CD4+ T-cell help is required for effective CD8+ T cell-mediated resolution of acute viral hepatitis in mice
- PMID: 24466045
- PMCID: PMC3897723
- DOI: 10.1371/journal.pone.0086348
CD4+ T-cell help is required for effective CD8+ T cell-mediated resolution of acute viral hepatitis in mice
Abstract
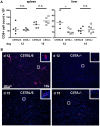
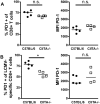
Cytotoxic CD8+ T cells are essential for the control of viral liver infections, such as those caused by HBV or HCV. It is not entirely clear whether CD4+ T-cell help is necessary for establishing anti-viral CD8+ T cell responses that successfully control liver infection. To address the role of CD4+ T cells in acute viral hepatitis, we infected mice with Lymphocytic Choriomeningitis Virus (LCMV) of the strain WE; LCMV-WE causes acute hepatitis in mice and is cleared from the liver by CD8+ T cells within about two weeks. The role of CD4+ T-cell help was studied in CD4+ T cell-lymphopenic mice, which were either induced by genetic deficiency of the major histocompatibility (MHC) class II transactivator (CIITA) in CIITA-/- mice, or by antibody-mediated CD4+ cell depletion. We found that CD4+ T cell-lymphopenic mice developed protracted viral liver infection, which seemed to be a consequence of reduced virus-specific CD8+ T-cell numbers in the liver. Moreover, the anti-viral effector functions of the liver-infiltrating CD8+ T cells in response to stimulation with LCMV peptide, notably the IFN-γ production and degranulation capacity were impaired in CIITA-/- mice. The impaired CD8+ T-cell function in CIITA-/- mice was not associated with increased expression of the exhaustion marker PD-1. Our findings indicate that CD4+ T-cell help is required to establish an effective antiviral CD8+ T-cell response in the liver during acute viral infection. Insufficient virus control and protracted viral hepatitis may be consequences of impaired initial CD4+ T-cell help.
Conflict of interest statement
Figures






References
-
- Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F (2013) The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 58(3): 593–608. - PubMed
-
- Ganem D, Prince AM (2004) Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 350: 1118–1129. - PubMed
-
- Lauer GM, Walker BD (2001) Hepatitis C virus infection. N Engl J Med 345: 41–52. - PubMed
-
- Hughes SA, Wedemeyer H, Harrison PM (2011) Hepatitis delta virus. Lancet 378: 73–85. - PubMed
-
- Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, et al. (2012) Hepatitis E. Lancet. 379: 2477–2488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

