Endocytosis of Fgf8 is a double-stage process and regulates spreading and signaling
- PMID: 24466061
- PMCID: PMC3896487
- DOI: 10.1371/journal.pone.0086373
Endocytosis of Fgf8 is a double-stage process and regulates spreading and signaling
Abstract
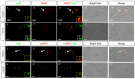
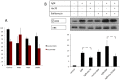
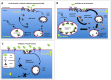
Tightly controlled concentration gradients of morphogens provide positional information and thus regulate tissue differentiation and morphogenesis in multicellular organisms. However, how such morphogenetic fields are formed and maintained remains debated. Here we show that fibroblast growth factor 8 (Fgf8) morphogen gradients in zebrafish embryos are established and maintained by two essential mechanisms. Firstly, Fgf8 is taken up into the cell by clathrin-mediated endocytosis. The speed of the uptake rate defines the range of the morphogenetic gradient of Fgf8. Secondly, our data demonstrate that after endocytosis the routing of Fgf8 from the early endosome to the late endosome shuts down signaling. Therefore, intracellular endocytic transport regulates the intensity and duration of Fgf8 signaling. We show that internalization of Fgf8 into the early endosome and subsequent transport towards the late endosome are two independent processes. Therefore, we hypothesize that Fgf8 receiving cells control both, the propagation width and the signal strength of the morphogen.
Conflict of interest statement
Figures




References
-
- Wiedlocha A, Sorensen V (2004) Signaling, internalization, and intracellular activity of fibroblast growth factor. Current topics in microbiology and immunology 286: 45–79. - PubMed
-
- Bokel C, Brand M (2013) Generation and interpretation of FGF morphogen gradients in vertebrates. Current opinion in genetics & development 23: 415–422. - PubMed
-
- Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, et al. (2000) Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Molecular cell 6: 743–750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

