Arsenic trioxide induces apoptosis in human platelets via C-Jun NH2-terminal kinase activation
- PMID: 24466103
- PMCID: PMC3899281
- DOI: 10.1371/journal.pone.0086445
Arsenic trioxide induces apoptosis in human platelets via C-Jun NH2-terminal kinase activation
Abstract
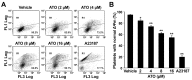
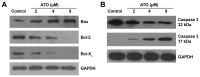
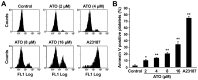
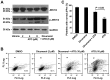
Arsenic trioxide (ATO), one of the oldest drugs in both Western and traditional Chinese medicine, has become an effective anticancer drug, especially in the treatment of acute promyelocytic leukemia (APL). However, thrombocytopenia occurred in most of ATO-treated patients with APL or other malignant diseases, and the pathogenesis remains unclear. Here we show that ATO dose-dependently induces depolarization of mitochondrial inner transmembrane potential (ΔΨm), up-regulation of Bax and down-regulation of Bcl-2 and Bcl-XL, caspase-3 activation, and phosphotidylserine (PS) exposure in platelets. ATO did not induce surface expression of P-selectin and PAC-1 binding, whereas, obviously reduced collagen, ADP, and thrombin induced platelet aggregation. ATO dose-dependently induced c-Jun NH2-terminal kinase (JNK) activation, and JNK specific inhibitor dicumarol obviously reduced ATO-induced ΔΨm depolarization in platelets. Clinical therapeutic dosage of ATO was intraperitoneally injected into C57 mice, and the numbers of circulating platelets were significantly reduced after five days of continuous injection. The data demonstrate that ATO induces caspase-dependent apoptosis via JNK activation in platelets. ATO does not incur platelet activation, whereas, it not only impairs platelet function but also reduces circulating platelets in vivo, suggesting the possible pathogenesis of thrombocytopenia in patients treated with ATO.
Conflict of interest statement
Figures


Similar articles
-
Carmustine induces platelet apoptosis.Platelets. 2015;26(5):437-42. doi: 10.3109/09537104.2014.928676. Epub 2014 Jun 23. Platelets. 2015. PMID: 24955606
-
A combination of sulindac and arsenic trioxide synergistically induces apoptosis in human lung cancer H1299 cells via c-Jun NH2-terminal kinase-dependent Bcl-xL phosphorylation.Lung Cancer. 2008 Sep;61(3):317-27. doi: 10.1016/j.lungcan.2008.01.002. Epub 2008 Feb 20. Lung Cancer. 2008. PMID: 18281123
-
Induction of B-chronic lymphocytic leukemia cell apoptosis by arsenic trioxide involves suppression of the phosphoinositide 3-kinase/Akt survival pathway via c-jun-NH2 terminal kinase activation and PTEN upregulation.Clin Cancer Res. 2010 Sep 1;16(17):4382-91. doi: 10.1158/1078-0432.CCR-10-0072. Epub 2010 Jun 9. Clin Cancer Res. 2010. PMID: 20534739
-
Arsenic-induced apoptosis in malignant cells in vitro.Leuk Lymphoma. 2000 Mar;37(1-2):53-63. doi: 10.3109/10428190009057628. Leuk Lymphoma. 2000. PMID: 10721769 Review.
-
Molecular targets of arsenic trioxide in malignant cells.Oncologist. 2002;7 Suppl 1:14-9. doi: 10.1634/theoncologist.7-suppl_1-14. Oncologist. 2002. PMID: 11961205 Review.
Cited by
-
Decreased cyclooxygenase-2 associated with impaired megakaryopoiesis and thrombopoiesis in primary immune thrombocytopenia.J Transl Med. 2023 Aug 12;21(1):540. doi: 10.1186/s12967-023-04389-9. J Transl Med. 2023. PMID: 37573325 Free PMC article.
-
Coenzyme Q10 protected against arsenite and enhanced the capacity of 2,3-dimercaptosuccinic acid to ameliorate arsenite-induced toxicity in mice.BMC Pharmacol Toxicol. 2021 Apr 7;22(1):19. doi: 10.1186/s40360-021-00484-z. BMC Pharmacol Toxicol. 2021. PMID: 33827703 Free PMC article.
-
Platelet apoptosis in patients with acute coronary syndromes.J Thromb Thrombolysis. 2015 May;39(4):539-46. doi: 10.1007/s11239-014-1160-8. J Thromb Thrombolysis. 2015. PMID: 25515895
-
Effect of Therapeutically Related Drugs on Coagulation-Anticoagulation Balance in Acute Promyelocytic Leukemia.Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221080166. doi: 10.1177/10760296221080166. Clin Appl Thromb Hemost. 2022. PMID: 35187963 Free PMC article. Review.
References
-
- Wang ZY, Chen Z (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111: 2505–2515. - PubMed
-
- Cohen MH, Hirschfeld S, Honig SF, Ibrahim A, Johnson JR, et al. (2001) Drug Approval Summaries: Arsenic Trioxide, Tamoxifen Citrate, Anastrazole, Paclitaxel, Bexarotene. The oncologist 6: 4–11. - PubMed
-
- Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, et al. (2007) Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs 25: 77–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

