Increased adenovirus Type 5 mediated transgene expression due to RhoB down-regulation
- PMID: 24466204
- PMCID: PMC3899303
- DOI: 10.1371/journal.pone.0086698
Increased adenovirus Type 5 mediated transgene expression due to RhoB down-regulation
Abstract
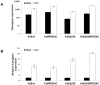
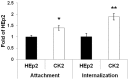
Adenovirus type 5 (Ad5) is a non-enveloped DNA virus frequently used as a gene transfer vector. Efficient Ad5 cell entry depends on the availability of its primary receptor, coxsackie and adenovirus receptor, which is responsible for attachment, and integrins, secondary receptors responsible for adenovirus internalization via clathrin-mediated endocytosis. However, efficacious adenovirus-mediated transgene expression also depends on successful trafficking of Ad5 particles to the nucleus of the target cell. It has been shown that changes occurring in tumor cells during development of resistance to anticancer drugs can be beneficial for adenovirus mediated transgene expression. In this study, using an in vitro model consisting of a parental cell line, human laryngeal carcinoma HEp2 cells, and a cisplatin-resistant clone CK2, we investigated the cause of increased Ad5-mediated transgene expression in CK2 as compared to HEp2 cells. We show that the primary cause of increased Ad5-mediated transgene expression in CK2 cells is not modulation of receptors on the cell surface or change in Ad5wt attachment and/or internalization, but is rather the consequence of decreased RhoB expression. We propose that RhoB plays an important role in Ad5 post-internalization events and more particularly in Ad5 intracellular trafficking. To the best of our knowledge, this is the first study showing changed Ad5 trafficking pattern between cells expressing different amount of RhoB, indicating the role of RhoB in Ad5 intracellular trafficking.
Conflict of interest statement
Figures




Similar articles
-
Cell-Surface Integrins and CAR Are Both Essential for Adenovirus Type 5 Transduction of Canine Cells of Lymphocytic Origin.PLoS One. 2017 Jan 9;12(1):e0169532. doi: 10.1371/journal.pone.0169532. eCollection 2017. PLoS One. 2017. PMID: 28068367 Free PMC article.
-
Pseudotyped αvβ6 integrin-targeted adenovirus vectors for ovarian cancer therapies.Oncotarget. 2016 May 10;7(19):27926-37. doi: 10.18632/oncotarget.8545. Oncotarget. 2016. PMID: 27056886 Free PMC article.
-
Adenovirus vectors based on human adenovirus type 19a have high potential for human muscle-directed gene therapy.Hum Gene Ther. 2006 Feb;17(2):193-205. doi: 10.1089/hum.2006.17.193. Hum Gene Ther. 2006. PMID: 16454653
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
Cited by
-
The Tongue Squamous Carcinoma Cell Line Cal27 Primarily Employs Integrin α6β4-Containing Type II Hemidesmosomes for Adhesion Which Contribute to Anticancer Drug Sensitivity.Front Cell Dev Biol. 2021 Dec 16;9:786758. doi: 10.3389/fcell.2021.786758. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977030 Free PMC article.
-
The Revolving Door of Adenovirus Cell Entry: Not All Pathways Are Equal.Pharmaceutics. 2021 Sep 29;13(10):1585. doi: 10.3390/pharmaceutics13101585. Pharmaceutics. 2021. PMID: 34683878 Free PMC article. Review.
References
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, et al. (1997) Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275: 1320–1323. - PubMed
-
- Davison E, Kirby I, Whitehouse J, Hart I, Marshall JF, et al. (2001) Adenovirus type 5 uptake by lung adenocarcinoma cells in culture correlates with Ad5 fibre binding is mediated by alpha(v)beta1 integrin and can be modulated by changes in beta1 integrin function. J Gene Med 3: 550–559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

