Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit
- PMID: 24466412
- PMCID: PMC3899560
- DOI: 10.1038/bonekey.2013.215
Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit
Abstract
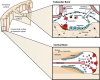
Coupling between bone formation and bone resorption refers to the process within basic multicellular units in which resorption by osteoclasts is met by the generation of osteoblasts from precursors, and their bone-forming activity, which needs to be sufficient to replace the bone lost. There are many sources of activities that contribute to coupling at remodeling sites, including growth factors released from the matrix, soluble and membrane products of osteoclasts and their precursors, signals from osteocytes and from immune cells and signaling taking place within the osteoblast lineage. Coupling is therefore a process that involves the interaction of a wide range of cell types and control mechanisms. As bone remodeling occurs at many sites asynchronously throughout the skeleton, locally generated activities comprise very important control mechanisms. In this review, we explore the potential roles of a number of these factors, including sphingosine-1-phosphate, semaphorins, ephrins, interleukin-6 (IL-6) family cytokines and marrow-derived factors. Their interactions achieve the essential tight control of coupling within individual remodeling units that is required for control of skeletal mass.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Frost HM. Dynamics of bone remodeling. Bone Biodyn 1964;315–333 (book chapter).
-
- Parfitt A. Morphological basis of bone mineral measurements: transient and steady state effects of treatment in osteoporosis. Miner Elecrolyte Metab 1980;4:273–287.
-
- Parfitt AM. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 1982;4:1–6. - PubMed
-
- Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev 1986;7:379–408. - PubMed
-
- Martin TJ, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr 2009;19:73–88. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
