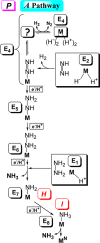
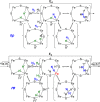
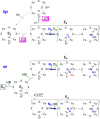
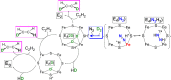
Mechanism of nitrogen fixation by nitrogenase: the next stage
- PMID: 24467365
- PMCID: PMC4012840
- DOI: 10.1021/cr400641x
Mechanism of nitrogen fixation by nitrogenase: the next stage
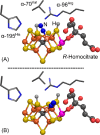
Figures
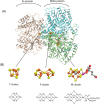
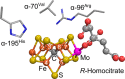
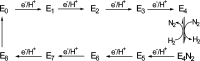
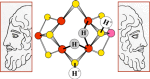
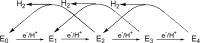
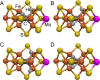
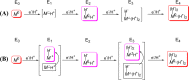
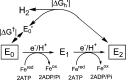
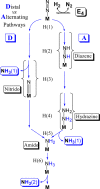
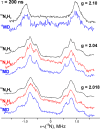
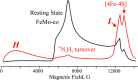
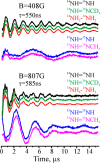

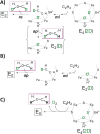
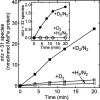
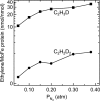
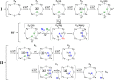
References
-
- Ferguson S. J. Curr. Opin. Chem. Biol. 1998, 2, 182. - PubMed
-
- Smil V.Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production; MIT Press: Cambridge, MA, 2004.
-
- Jia H.-P.; Quadrelli E. A. Chem. Soc. Rev. 2014, 43, 547. - PubMed
-
- MacKay B. A.; Fryzuk M. D. Chem. Rev. 2004, 104, 385. - PubMed
-
- Canfield D. E.; Glazer A. N.; Falkowski P. G. Science 2010, 330, 192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

