The mononuclear molybdenum enzymes
- PMID: 24467397
- PMCID: PMC4080432
- DOI: 10.1021/cr400443z
The mononuclear molybdenum enzymes
Conflict of interest statement
The authors declare no competing financial interest.
Figures












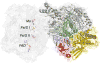


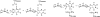


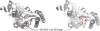
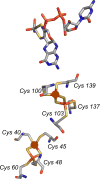

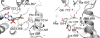
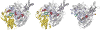





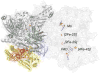


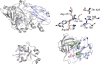

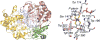




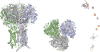

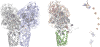




References
-
- Derenzo EC, Heytler PG, Kaleita E. Arch Biochem Biophys. 1954;49:242. - PubMed
- Mahler HR, Mackler B, Green DE, Bock RM. J Biol Chem. 1954;210:465. - PubMed
- Nicholas DJD, Nason A, McElroy WD. J Biol Chem. 1954;207:341. - PubMed
- Cohen HJ, Fridovich I, Rajagopalan K. J Biol Chem. 1971;246:374. - PubMed
- Richert DA, Westerfeld WW. J Biol Chem. 1953;203:915. - PubMed
-
- Cvetkovic A, Menon AL, Thorgersen MP, Scott JW, Poole FL, II, Jenney FE, Jr, Lancaster WA, Praissman JL, Shanmukh S, Vaccaro BJ, Trauger SA, Kalisiak E, Apon JV, Siuzdak G, Yannone SM, Tainer JA, Adams MWW. Nature. 2010;466:779. - PubMed
- Zhang Y, Gladyshev VN. J Biol Chem. 2011;286:23623. - PMC - PubMed
- Zhang Y, Rump S, Gladyshev VN. Coord Chem Rev. 2011;255:1206. - PMC - PubMed
-
- Hille R. Chem Rev. 1996;96:2757. - PubMed
-
- Johnson MK, Rees DC, Adams MWW. Chem Rev. 1996;96:2817. - PubMed
-
- Chan MK, Mukund S, Kletzin A, Adams MWW, Rees DC. Science. 1995;267:1463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

