Arylamine N-acetyltransferases: from drug metabolism and pharmacogenetics to drug discovery
- PMID: 24467436
- PMCID: PMC4158862
- DOI: 10.1111/bph.12598
Arylamine N-acetyltransferases: from drug metabolism and pharmacogenetics to drug discovery
Abstract
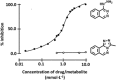
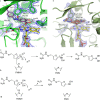
Arylamine N-acetyltransferases (NATs) are polymorphic drug-metabolizing enzymes, acetylating arylamine carcinogens and drugs including hydralazine and sulphonamides. The slow NAT phenotype increases susceptibility to hydralazine and isoniazid toxicity and to occupational bladder cancer. The two polymorphic human NAT loci show linkage disequilibrium. All mammalian Nat genes have an intronless open reading frame and non-coding exons. The human gene products NAT1 and NAT2 have distinct substrate specificities: NAT2 acetylates hydralazine and human NAT1 acetylates p-aminosalicylate (p-AS) and the folate catabolite para-aminobenzoylglutamate (p-abaglu). Human NAT2 is mainly in liver and gut. Human NAT1 and its murine homologue are in many adult tissues and in early embryos. Human NAT1 is strongly expressed in oestrogen receptor-positive breast cancer and may contribute to folate and acetyl CoA homeostasis. NAT enzymes act through a catalytic triad of Cys, His and Asp with the architecture of the active site-modulating specificity. Polymorphisms may cause unfolded protein. The C-terminus helps bind acetyl CoA and differs among NATs including prokaryotic homologues. NAT in Salmonella typhimurium supports carcinogen activation and NAT in mycobacteria metabolizes isoniazid with polymorphism a minor factor in isoniazid resistance. Importantly, nat is in a gene cluster essential for Mycobacterium tuberculosis survival inside macrophages. NAT inhibitors are a starting point for novel anti-tuberculosis drugs. Human NAT1-specific inhibitors may act in biomarker detection in breast cancer and in cancer therapy. NAT inhibitors for co-administration with 5-aminosalicylate (5-AS) in inflammatory bowel disease has prompted ongoing investigations of azoreductases in gut bacteria which release 5-AS from prodrugs including balsalazide.
Keywords: acetyl CoA; arylamine N-acetyltransferase; breast cancer; catalytic triad; hydralazine; isoniazid; pharmacogenetics; tuberculosis.
© 2014 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures






References
-
- Abuhammad A, Lack N, Schweichler J, Staunton D, Sim RB, Sim E. Improvement of the expression and purification of Mycobacterium tuberculosis arylamine N-acetyltransferase (TBNAT) a potential target for novel anti-tubercular agents. Protein Expr Purif. 2011;80:246–252. - PubMed
-
- Abuhammad A, Fullam E, Lowe ED, Staunton D, Kawamura A, Sim E. Piperidinols that show anti-tubercular activity are inhibitors of arylamine N-acetyltransferase: an essential enzyme for mycobacterial survival inside macrophages. PLoS ONE. 2012;7:e52790. doi: 10.1371/journal.pone.0052790. - DOI - PMC - PubMed
-
- Abuhammad A, Lowe ED, McDonough MA, Shaw Stewart PD, Kolek SA, Sim E, Garman EF. Structure of arylamine N-acetyltransferase from Mycobacterium tuberculosis determined by cross-seeding with the homologous protein from M. marinum: triumph over adversity. Acta Crystallogr D Biol Crystallogr. 2013;69:1433–1446. - PubMed
-
- Adam PJ, Berry J, Loader JA, Tyson KL, Craggs G, Smith P, et al. NAT-1 is highly expressed in breast cancers and conveys enhanced growth resistance to etoposide in vitro. Mol Cancer Res. 2003;1:826–835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

