The dual PPARα/γ agonist aleglitazar increases the number and function of endothelial progenitor cells: implications for vascular function and atherogenesis
- PMID: 24467636
- PMCID: PMC4009009
- DOI: 10.1111/bph.12608
The dual PPARα/γ agonist aleglitazar increases the number and function of endothelial progenitor cells: implications for vascular function and atherogenesis
Abstract
Background and purpose: Aleglitazar is a dual PPARα/γ agonist but little is known about its effects on vascular function and atherogenesis. Hence, we characterized its effects on circulating angiogenic cells (CAC), neoangiogenesis, endothelial function, arteriogenesis and atherosclerosis in mice.
Experimental approach: C57Bl/6 wild-type (WT, normal chow), endothelial NOS (eNOS)(-/-) (normal chow) and ApoE(-/-) (Western-type diet) mice were treated with aleglitazar (10 mg·kg(-1) ·day(-1) , i.p.) or vehicle.
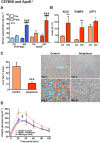
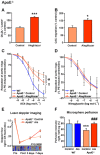
Key results: Aleglitazar enhanced expression of PPARα and PPARγ target genes, normalized glucose tolerance and potently reduced hepatic fat in ApoE(-/-) mice. In WT mice, but not in eNOS(-/-) , aleglitazar up-regulated Sca-1/VEGFR2-positive CAC in the blood and bone marrow and up-regulated diLDL/lectin-positive CAC. Aleglitazar augmented CAC migration and enhanced neoangiogenesis. In ApoE(-/-) mice, aleglitazar up-regulated CAC number and function, reduced markers of vascular inflammation and potently improved perfusion restoration after hindlimb ischaemia and aortic endothelium-dependent vasodilatation. This was associated with markedly reduced formation of atherosclerotic plaques. In human cultured CAC from healthy donors and patients with coronary artery disease with or without diabetes mellitus, aleglitazar increased migration and colony-forming units in a concentration-dependent manner. Furthermore, oxidative stress-induced CAC apoptosis and expression of p53 were reduced, while telomerase activity and expression of phospho-eNOS and phospho-Akt were elevated. Comparative agonist and inhibitor experiments revealed that aleglitazar's effects on CAC migration and colony-forming units were mediated by both PPARα and PPARγ signalling and required Akt.
Conclusions and implications: Aleglitazar augments the number, function and survival of CAC, which correlates with improved vascular function, enhanced arteriogenesis and prevention of atherosclerosis in mice.
Keywords: PPARα/γ agonist; aleglitazar; arteriogenesis; atherosclerosis; circulating angiogenic cells; endothelial NO synthase; endothelial function; endothelial progenitor cells.
© 2014 The British Pharmacological Society.
Figures






Similar articles
-
Aleglitazar, a Balanced Dual PPARα and -γ Agonist, Protects the Heart Against Ischemia-Reperfusion Injury.Cardiovasc Drugs Ther. 2016 Apr;30(2):129-41. doi: 10.1007/s10557-016-6650-9. Cardiovasc Drugs Ther. 2016. PMID: 26861490
-
Dual PPARα/γ agonist aleglitazar confers stroke protection in a model of mild focal brain ischemia in mice.J Mol Med (Berl). 2019 Aug;97(8):1127-1138. doi: 10.1007/s00109-019-01801-0. Epub 2019 May 30. J Mol Med (Berl). 2019. PMID: 31147725 Free PMC article.
-
BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis.J Thorac Cardiovasc Surg. 2013 Oct;146(4):949-960.e4. doi: 10.1016/j.jtcvs.2012.12.064. Epub 2013 Feb 14. J Thorac Cardiovasc Surg. 2013. PMID: 23415688
-
Aleglitazar, a dual PPARα and PPARγ agonist for the potential oral treatment of type 2 diabetes mellitus.IDrugs. 2010 Nov;13(11):793-801. IDrugs. 2010. PMID: 21046527 Review.
-
Cardiovascular Risk and Safety Evaluation of a Dual Peroxisome Proliferator-Activated Receptor-Alpha/Gamma Agonist, Aleglitazar, in Patients With Type 2 Diabetes: A Meta-analysis.J Cardiovasc Pharmacol. 2020 Apr;75(4):351-357. doi: 10.1097/FJC.0000000000000796. J Cardiovasc Pharmacol. 2020. PMID: 31929323 Review.
Cited by
-
A comparative pharmacogenomic analysis of three classic TCM prescriptions for coronary heart disease based on molecular network modeling.Acta Pharmacol Sin. 2020 Jun;41(6):735-744. doi: 10.1038/s41401-019-0352-3. Epub 2020 Feb 12. Acta Pharmacol Sin. 2020. PMID: 32051552 Free PMC article.
-
Peroxisome Proliferator-Activated Receptor α/γ and Cannabinoid Receptor 2 Agonist Attenuated Nonalcoholic Steatohepatitis Exosome-Related Abnormalities in Mice.Am J Pathol. 2025 Feb;195(2):188-203. doi: 10.1016/j.ajpath.2024.10.006. Epub 2024 Oct 26. Am J Pathol. 2025. PMID: 39490440
-
Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice.Cardiovasc Diabetol. 2016 Jun 18;15:88. doi: 10.1186/s12933-016-0408-3. Cardiovasc Diabetol. 2016. PMID: 27316923 Free PMC article.
-
MicroRNA-27a promotes renal tubulointerstitial fibrosis via suppressing PPARγ pathway in diabetic nephropathy.Oncotarget. 2016 Jul 26;7(30):47760-47776. doi: 10.18632/oncotarget.10283. Oncotarget. 2016. PMID: 27351287 Free PMC article.
-
SIRT3 attenuates coronary atherosclerosis in diabetic patients by regulating endothelial cell function.J Clin Lab Anal. 2022 Aug;36(8):e24586. doi: 10.1002/jcla.24586. Epub 2022 Jul 6. J Clin Lab Anal. 2022. PMID: 35791925 Free PMC article.
References
-
- Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, et al. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol. 2004;24:684–690. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Benardeau A, Benz J, Binggeli A, Blum D, Boehringer M, Grether U, et al. Aleglitazar, a new, potent, and balanced dual PPAR alpha/gamma agonist for the treatment of type II diabetes. Bioorg Med Chem Lett. 2009;19:2468–2473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

