Sertolin mediates blood-testis barrier restructuring
- PMID: 24467744
- PMCID: PMC3959606
- DOI: 10.1210/en.2013-1850
Sertolin mediates blood-testis barrier restructuring
Abstract
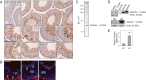
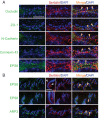
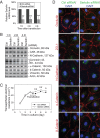
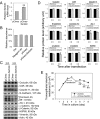
Two important events that occur during mammalian spermatogenesis are the release of elongated spermatids at late stage VIII of the seminiferous epithelial cycle and the restructuring of the blood-testis barrier (BTB) during stages VIII-XI. Still, it is not completely understood how these cellular events are accomplished within the seminiferous epithelium. In the present study, we investigate how sertolin, a protein that was initially identified, cloned, and partially characterized by our laboratory, functions in these critical events. Sertolin was found at the BTB, as well as at the apical ectoplasmic specialization and apical tubulobulbar complex, where it colocalized with epidermal growth factor receptor kinase substrate 8 and actin-related protein 3, two actin-regulatory proteins. Knockdown of sertolin by RNA interference showed Sertoli cell barrier function to be enhanced when assessed by transepithelial electrical resistance measurements and immunolocalization experiments. By contrast, the integrity of the BTB was disrupted when sertolin was overexpressed in vitro and in vivo. Sertolin overexpression also prompted germ cell loss from the seminiferous epithelium. Taken collectively, these results suggest that sertolin may be involved in coordinating spermatid release and BTB restructuring during spermatogenesis in the rat.
Figures
Similar articles
-
c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes.Int J Biochem Cell Biol. 2011 Apr;43(4):651-65. doi: 10.1016/j.biocel.2011.01.008. Epub 2011 Jan 21. Int J Biochem Cell Biol. 2011. PMID: 21256972 Free PMC article.
-
Annexin A2 is critical for blood-testis barrier integrity and spermatid disengagement in the mammalian testis.Biochim Biophys Acta Mol Cell Res. 2017 Mar;1864(3):527-545. doi: 10.1016/j.bbamcr.2016.12.012. Epub 2016 Dec 11. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27974247
-
Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model.J Cell Physiol. 2005 Oct;205(1):141-57. doi: 10.1002/jcp.20377. J Cell Physiol. 2005. PMID: 15880438
-
Intercellular adhesion molecules (ICAMs) and spermatogenesis.Hum Reprod Update. 2013 Mar-Apr;19(2):167-86. doi: 10.1093/humupd/dms049. Epub 2013 Jan 3. Hum Reprod Update. 2013. PMID: 23287428 Free PMC article. Review.
-
Transcriptional regulation of cell adhesion at the blood-testis barrier and spermatogenesis in the testis.Adv Exp Med Biol. 2012;763:281-94. doi: 10.1007/978-1-4614-4711-5_14. Adv Exp Med Biol. 2012. PMID: 23397630 Free PMC article. Review.
Cited by
-
Establishment and functional characterization of a murine primary Sertoli cell line deficient of connexin43.Cell Tissue Res. 2020 Aug;381(2):309-326. doi: 10.1007/s00441-020-03203-y. Epub 2020 Apr 23. Cell Tissue Res. 2020. PMID: 32328805 Free PMC article.
-
The relationship between sperm secretion and the Hippo signaling pathway in the rat seminiferous tubules.Sci Rep. 2025 Aug 2;15(1):28204. doi: 10.1038/s41598-025-12819-5. Sci Rep. 2025. PMID: 40753289 Free PMC article.
References
-
- de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, eds. The Physiology of Reproduction. New York, NY: Raven Press; 1988:837–932
-
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

