Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation
- PMID: 24467809
- PMCID: PMC3978694
- DOI: 10.1186/ar4456
Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation
Abstract
Introduction: We previously reported that sialyl Lewis(y), synthesized by fucosyltransferases, is involved in angiogenesis. Fucosyltransferase 1 (fut1) is an α(1,2)-fucosyltransferase responsible for synthesis of the H blood group and Lewis(y) antigens. However, the angiogenic involvement of fut 1 in the pathogenesis of rheumatoid arthritis synovial tissue (RA ST) has not been clearly defined.
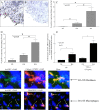
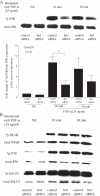
Methods: Assay of α(1,2)-linked fucosylated proteins in RA was performed by enzyme-linked lectin assay. Fut1 expression was determined in RA ST samples by immunohistological staining. We performed angiogenic Matrigel assays using a co-culture system of human dermal microvascular endothelial cells (HMVECs) and fut1 small interfering RNA (siRNA) transfected RA synovial fibroblasts. To determine if fut1 played a role in leukocyte retention and cell proliferation in the RA synovium, myeloid THP-1 cell adhesion assays and fut1 siRNA transfected RA synovial fibroblast proliferation assays were performed.
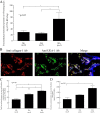
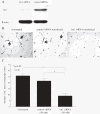
Results: Total α(1,2)-linked fucosylated proteins in RA ST were significantly higher compared to normal (NL) ST. Fut1 expression on RA ST lining cells positively correlated with ST inflammation. HMVECs from a co-culture system with fut1 siRNA transfected RA synovial fibroblasts exhibited decreased endothelial cell tube formation compared to control siRNA transfected RA synovial fibroblasts. Fut1 siRNA also inhibited myeloid THP-1 adhesion to RA synovial fibroblasts and RA synovial fibroblast proliferation.
Conclusions: These data show that α(1,2)-linked fucosylated proteins are upregulated in RA ST compared to NL ST. We also show that fut1 in RA synovial fibroblasts is important in angiogenesis, leukocyte-synovial fibroblast adhesion, and synovial fibroblast proliferation, all key processes in the pathogenesis of RA.
Figures
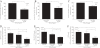

Similar articles
-
Fucosyltransferase 1 mediates angiogenesis in rheumatoid arthritis.Arthritis Rheumatol. 2014 Aug;66(8):2047-58. doi: 10.1002/art.38648. Arthritis Rheumatol. 2014. PMID: 24692243 Free PMC article.
-
ADAM-10 is overexpressed in rheumatoid arthritis synovial tissue and mediates angiogenesis.Arthritis Rheum. 2013 Jan;65(1):98-108. doi: 10.1002/art.37755. Arthritis Rheum. 2013. PMID: 23124962
-
Inflammatory properties of inhibitor of DNA binding 1 secreted by synovial fibroblasts in rheumatoid arthritis.Arthritis Res Ther. 2016 Apr 12;18:87. doi: 10.1186/s13075-016-0984-3. Arthritis Res Ther. 2016. PMID: 27071670 Free PMC article.
-
The pathogenic role of angiogenesis in rheumatoid arthritis.Angiogenesis. 2015 Oct;18(4):433-48. doi: 10.1007/s10456-015-9477-2. Epub 2015 Jul 22. Angiogenesis. 2015. PMID: 26198292 Free PMC article. Review.
-
The metastasis associated protein S100A4: a potential novel link to inflammation and consequent aggressive behaviour of rheumatoid arthritis synovial fibroblasts.Ann Rheum Dis. 2008 Nov;67(11):1499-504. doi: 10.1136/ard.2007.079905. Epub 2007 Dec 4. Ann Rheum Dis. 2008. PMID: 18056757 Review.
Cited by
-
Role of Long Intergenic Nonprotein-Coding RNA 00511 in Nod-Like Receptor Protein Pyrin Domain 3-Induced Chondrocyte Pyroptosis via the MicroRNA-9-5p/FUT1 Axis.J Microbiol Biotechnol. 2024 Jul 28;34(7):1511-1521. doi: 10.4014/jmb.2312.12014. Epub 2024 Apr 15. J Microbiol Biotechnol. 2024. PMID: 38934781 Free PMC article.
-
Association between the ABO blood group and primary knee osteoarthritis: A case-control study.J Orthop Translat. 2019 Sep 5;21:129-135. doi: 10.1016/j.jot.2019.08.003. eCollection 2020 Mar. J Orthop Translat. 2019. PMID: 32309138 Free PMC article.
-
MicroRNA-101-3p inhibits fibroblast-like synoviocyte proliferation and inflammation in rheumatoid arthritis by targeting PTGS2.Biosci Rep. 2020 Jan 31;40(1):BSR20191136. doi: 10.1042/BSR20191136. Biosci Rep. 2020. Retraction in: Biosci Rep. 2020 Aug 28;40(8):BSR-20191136_RET. doi: 10.1042/BSR-20191136_RET. PMID: 31894846 Free PMC article. Retracted.
-
Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells.Cell Death Discov. 2019 Mar 6;5:74. doi: 10.1038/s41420-019-0145-y. eCollection 2019. Cell Death Discov. 2019. PMID: 30854233 Free PMC article.
-
High-density genotyping of immune loci in Kawasaki disease and IVIG treatment response in European-American case-parent trio study.Genes Immun. 2014 Dec;15(8):534-42. doi: 10.1038/gene.2014.47. Epub 2014 Aug 7. Genes Immun. 2014. PMID: 25101798 Free PMC article.
References
-
- Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, Strieter RM. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;16:2187–2195. - PubMed
-
- Kunkel SL, Lukacs N, Kasama T, Strieter RM. The role of chemokines in inflammatory joint disease. J Leukoc Biol. 1996;16:6–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

