Host matrix metalloproteinases in cerebral malaria: new kids on the block against blood-brain barrier integrity?
- PMID: 24467887
- PMCID: PMC3905658
- DOI: 10.1186/2045-8118-11-1
Host matrix metalloproteinases in cerebral malaria: new kids on the block against blood-brain barrier integrity?
Abstract
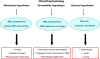
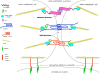
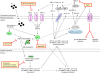
Cerebral malaria (CM) is a life-threatening complication of falciparum malaria, associated with high mortality rates, as well as neurological impairment in surviving patients. Despite disease severity, the etiology of CM remains elusive. Interestingly, although the Plasmodium parasite is sequestered in cerebral microvessels, it does not enter the brain parenchyma: so how does Plasmodium induce neuronal dysfunction? Several independent research groups have suggested a mechanism in which increased blood-brain barrier (BBB) permeability might allow toxic molecules from the parasite or the host to enter the brain. However, the reported severity of BBB damage in CM is variable depending on the model system, ranging from mild impairment to full BBB breakdown. Moreover, the factors responsible for increased BBB permeability are still unknown. Here we review the prevailing theories on CM pathophysiology and discuss new evidence from animal and human CM models implicating BBB damage. Finally, we will review the newly-described role of matrix metalloproteinases (MMPs) and BBB integrity. MMPs comprise a family of proteolytic enzymes involved in modulating inflammatory response, disrupting tight junctions, and degrading sub-endothelial basal lamina. As such, MMPs represent potential innovative drug targets for CM.
Figures


Similar articles
-
Brain Endothelium: The "Innate Immunity Response Hypothesis" in Cerebral Malaria Pathogenesis.Front Immunol. 2019 Jan 29;9:3100. doi: 10.3389/fimmu.2018.03100. eCollection 2018. Front Immunol. 2019. PMID: 30761156 Free PMC article. Review.
-
A new hypothesis on the manifestation of cerebral malaria: the secret is in the liver.Med Hypotheses. 2013 Nov;81(5):777-83. doi: 10.1016/j.mehy.2013.08.005. Epub 2013 Aug 13. Med Hypotheses. 2013. PMID: 23978689 Free PMC article.
-
Human cerebral malaria and the blood-brain barrier.Int J Parasitol. 2006 May 1;36(5):555-68. doi: 10.1016/j.ijpara.2006.02.004. Epub 2006 Mar 10. Int J Parasitol. 2006. PMID: 16616145 Review.
-
Metabolic acidosis induced by Plasmodium falciparum intraerythrocytic stages alters blood-brain barrier integrity.J Cereb Blood Flow Metab. 2011 Feb;31(2):514-26. doi: 10.1038/jcbfm.2010.121. Epub 2010 Aug 4. J Cereb Blood Flow Metab. 2011. PMID: 20683453 Free PMC article.
-
Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury.J Neurochem. 2012 Jan;120(1):147-56. doi: 10.1111/j.1471-4159.2011.07542.x. Epub 2011 Nov 17. J Neurochem. 2012. PMID: 22007835
Cited by
-
Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis.Parasitology. 2019 Mar;146(3):284-298. doi: 10.1017/S0031182018001464. Epub 2018 Sep 24. Parasitology. 2019. PMID: 30246668 Free PMC article.
-
Cerebral Malaria Model Applying Human Brain Organoids.Cells. 2023 Mar 23;12(7):984. doi: 10.3390/cells12070984. Cells. 2023. PMID: 37048057 Free PMC article.
-
The Role of Tissue Inhibitor of Metalloproteinase-1 and 2 in Echinococcus granulosus senso lato-Induced Human Hepatic Fibrosis.Acta Parasitol. 2022 Jun;67(2):851-857. doi: 10.1007/s11686-022-00534-4. Epub 2022 Mar 16. Acta Parasitol. 2022. PMID: 35294975
-
Pathogenesis of cerebral malaria--inflammation and cytoadherence.Front Cell Infect Microbiol. 2014 Jul 29;4:100. doi: 10.3389/fcimb.2014.00100. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25120958 Free PMC article. Review.
-
Cerebral Malaria and Neuronal Implications of Plasmodium Falciparum Infection: From Mechanisms to Advanced Models.Adv Sci (Weinh). 2022 Dec;9(36):e2202944. doi: 10.1002/advs.202202944. Epub 2022 Oct 27. Adv Sci (Weinh). 2022. PMID: 36300890 Free PMC article. Review.
References
-
- World Health Organization (WHO) World Malaria Report. Geneva: World Health Organization; 2013.
-
- Khadjavi A, Giribaldi G, Prato M. From control to eradication of malaria: the end of being stuck in second gear? Asian Pac J Trop Med. 2010;3:412–420.
-
- Mohanty S, Patel DK, Pati SS, Mishra SK. Adjuvant therapy in cerebral malaria. Indian J Med Res. 2006;124:245–260. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

