Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke?
- PMID: 24467911
- PMCID: PMC3961549
- DOI: 10.1016/j.pneurobio.2013.12.008
Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke?
Abstract
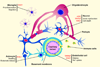
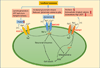
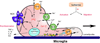
Ischemic brain injury results from complicated cellular mechanisms. The present therapy for acute ischemic stroke is limited to thrombolysis with the recombinant tissue plasminogen activator (rtPA) and mechanical recanalization. Therefore, a better understanding of ischemic brain injury is needed for the development of more effective therapies. Disruption of ionic homeostasis plays an important role in cell death following cerebral ischemia. Glutamate receptor-mediated ionic imbalance and neurotoxicity have been well established in cerebral ischemia after stroke. However, non-NMDA receptor-dependent mechanisms, involving acid-sensing ion channel 1a (ASIC1a), transient receptor potential melastatin 7 (TRPM7), and Na(+)/H(+) exchanger isoform 1 (NHE1), have recently emerged as important players in the dysregulation of ionic homeostasis in the CNS under ischemic conditions. These H(+)-sensitive channels and/or exchangers are expressed in the majority of cell types of the neurovascular unit. Sustained activation of these proteins causes excessive influx of cations, such as Ca(2+), Na(+), and Zn(2+), and leads to ischemic reperfusion brain injury. In this review, we summarize recent pre-clinical experimental research findings on how these channels/exchangers are regulated in both in vitro and in vivo models of cerebral ischemia. The blockade or transgenic knockdown of these proteins was shown to be neuroprotective in these ischemia models. Taken together, these non-NMDA receptor-dependent mechanisms may serve as novel therapeutic targets for stroke intervention.
Keywords: ASIC; Acidosis; Calcium; NHE1; TRPM7; Zinc.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


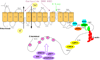
Similar articles
-
Disrupted Ionic Homeostasis in Ischemic Stroke and New Therapeutic Targets.J Stroke Cerebrovasc Dis. 2017 Dec;26(12):2706-2719. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.011. Epub 2017 Oct 18. J Stroke Cerebrovasc Dis. 2017. PMID: 29054733 Review.
-
Acid sensing ion channels--novel therapeutic targets for ischemic brain injury.Front Biosci. 2007 Jan 1;12:1376-86. doi: 10.2741/2154. Front Biosci. 2007. PMID: 17127388 Review.
-
β-Estradiol Protects Against Acidosis-Mediated and Ischemic Neuronal Injury by Promoting ASIC1a (Acid-Sensing Ion Channel 1a) Protein Degradation.Stroke. 2019 Oct;50(10):2902-2911. doi: 10.1161/STROKEAHA.119.025940. Epub 2019 Aug 15. Stroke. 2019. PMID: 31412757 Free PMC article.
-
Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation.PLoS One. 2014 Jan 2;9(1):e84294. doi: 10.1371/journal.pone.0084294. eCollection 2014. PLoS One. 2014. PMID: 24392123 Free PMC article.
-
Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia.Neurol Sci. 2012 Oct;33(5):1125-31. doi: 10.1007/s10072-011-0916-6. Epub 2012 Jan 10. Neurol Sci. 2012. PMID: 22231470
Cited by
-
Properties of acid-induced currents in mouse dorsal root ganglia neurons.Physiol Rep. 2016 May;4(9):e12795. doi: 10.14814/phy2.12795. Physiol Rep. 2016. PMID: 27173673 Free PMC article.
-
Commentary: Slowing of the Time Course of Acidification Decreases the Acid-Sensing Ion Channel 1a Current Amplitude and Modulates Action Potential Firing in Neurons.Front Cell Neurosci. 2021 Jul 16;15:714204. doi: 10.3389/fncel.2021.714204. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34335195 Free PMC article. No abstract available.
-
Ion Channel Dysregulation Following Intracerebral Hemorrhage.Neurosci Bull. 2024 Mar;40(3):401-414. doi: 10.1007/s12264-023-01118-6. Epub 2023 Sep 27. Neurosci Bull. 2024. PMID: 37755675 Free PMC article. Review.
-
Normobaric Hyperoxia Therapy in Treating Stroke.Clin Interv Aging. 2025 Jul 2;20:969-981. doi: 10.2147/CIA.S521584. eCollection 2025. Clin Interv Aging. 2025. PMID: 40625679 Free PMC article. Review.
-
Elevated Na/H exchanger 1 (SLC9A1) emerges as a marker for tumorigenesis and prognosis in gliomas.J Exp Clin Cancer Res. 2018 Oct 17;37(1):255. doi: 10.1186/s13046-018-0923-z. J Exp Clin Cancer Res. 2018. PMID: 30333031 Free PMC article.
References
-
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. - PubMed
-
- Abed E, Martineau C, Moreau R. Role of melastatin transient receptor potential 7 channels in the osteoblastic differentiation of murine MC3T3 cells. Calcif Tissue Int. 2011;88:246–253. - PubMed
-
- Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am J Physiol Cell Physiol. 2009;297:C360–C368. - PubMed
-
- Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

