Host insulin stimulates Echinococcus multilocularis insulin signalling pathways and larval development
- PMID: 24468049
- PMCID: PMC3923246
- DOI: 10.1186/1741-7007-12-5
Host insulin stimulates Echinococcus multilocularis insulin signalling pathways and larval development
Abstract
Background: The metacestode of the tapeworm Echinococcus multilocularis is the causative agent of alveolar echinococcosis, a lethal zoonosis. Infections are initiated through establishment of parasite larvae within the intermediate host's liver, where high concentrations of insulin are present, followed by tumour-like growth of the metacestode in host organs. The molecular mechanisms determining the organ tropism of E. multilocularis or the influences of host hormones on parasite proliferation are poorly understood.
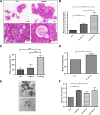
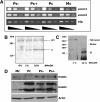
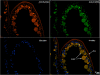
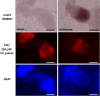
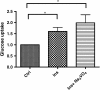
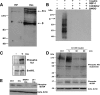
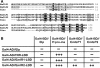
Results: Using in vitro cultivation systems for parasite larvae we show that physiological concentrations (10 nM) of human insulin significantly stimulate the formation of metacestode larvae from parasite stem cells and promote asexual growth of the metacestode. Addition of human insulin to parasite larvae led to increased glucose uptake and enhanced phosphorylation of Echinococcus insulin signalling components, including an insulin receptor-like kinase, EmIR1, for which we demonstrate predominant expression in the parasite's glycogen storage cells. We also characterized a second insulin receptor family member, EmIR2, and demonstrated interaction of its ligand binding domain with human insulin in the yeast two-hybrid system. Addition of an insulin receptor inhibitor resulted in metacestode killing, prevented metacestode development from parasite stem cells, and impaired the activation of insulin signalling pathways through host insulin.
Conclusions: Our data indicate that host insulin acts as a stimulant for parasite development within the host liver and that E. multilocularis senses the host hormone through an evolutionarily conserved insulin signalling pathway. Hormonal host-parasite cross-communication, facilitated by the relatively close phylogenetic relationship between E. multilocularis and its mammalian hosts, thus appears to be important in the pathology of alveolar echinococcosis. This contributes to a closer understanding of organ tropism and parasite persistence in larval cestode infections. Furthermore, our data show that Echinococcus insulin signalling pathways are promising targets for the development of novel drugs.
Figures
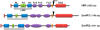



Similar articles
-
The role of fibroblast growth factor signalling in Echinococcus multilocularis development and host-parasite interaction.PLoS Negl Trop Dis. 2019 Mar 8;13(3):e0006959. doi: 10.1371/journal.pntd.0006959. eCollection 2019 Mar. PLoS Negl Trop Dis. 2019. PMID: 30849083 Free PMC article.
-
A MEKK1 - JNK mitogen activated kinase (MAPK) cascade module is active in Echinococcus multilocularis stem cells.PLoS Negl Trop Dis. 2021 Dec 8;15(12):e0010027. doi: 10.1371/journal.pntd.0010027. eCollection 2021 Dec. PLoS Negl Trop Dis. 2021. PMID: 34879059 Free PMC article.
-
Serotonin stimulates Echinococcus multilocularis larval development.Parasit Vectors. 2021 Jan 6;14(1):14. doi: 10.1186/s13071-020-04533-0. Parasit Vectors. 2021. PMID: 33407815 Free PMC article.
-
Echinococcus-Host Interactions at Cellular and Molecular Levels.Adv Parasitol. 2017;95:147-212. doi: 10.1016/bs.apar.2016.09.001. Epub 2016 Nov 21. Adv Parasitol. 2017. PMID: 28131363 Review.
-
The role of evolutionarily conserved signalling systems in Echinococcus multilocularis development and host-parasite interaction.Med Microbiol Immunol. 2010 Aug;199(3):247-59. doi: 10.1007/s00430-010-0154-1. Epub 2010 Apr 8. Med Microbiol Immunol. 2010. PMID: 20376483 Review.
Cited by
-
Metformin Suppresses Development of the Echinococcus multilocularis Larval Stage by Targeting the TOR Pathway.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01808-19. doi: 10.1128/AAC.01808-19. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32540980 Free PMC article.
-
Identification of hydatidosis-related modules and key regulatory genes.PeerJ. 2020 Jun 18;8:e9280. doi: 10.7717/peerj.9280. eCollection 2020. PeerJ. 2020. PMID: 32596042 Free PMC article.
-
Proliferative cells in racemose neurocysticercosis have an active MAPK signalling pathway and respond to metformin treatment.Int J Parasitol. 2022 May;52(6):377-383. doi: 10.1016/j.ijpara.2022.01.001. Epub 2022 Feb 17. Int J Parasitol. 2022. PMID: 35182540 Free PMC article.
-
Compound library screening identified Akt/PKB kinase pathway inhibitors as potential key molecules for the development of new chemotherapeutics against schistosomiasis.Int J Parasitol Drugs Drug Resist. 2014 Oct 12;4(3):256-66. doi: 10.1016/j.ijpddr.2014.09.004. eCollection 2014 Dec. Int J Parasitol Drugs Drug Resist. 2014. PMID: 25516836 Free PMC article.
-
Echinococcus multilocularis infection affects risk-taking behaviour in Microtus arvalis: adaptive manipulation?Parasitology. 2024 Jun;151(7):650-656. doi: 10.1017/S0031182024000507. Epub 2024 May 20. Parasitology. 2024. PMID: 38766838 Free PMC article.
References
-
- Misbin RI, Merimee TJ, Lowenstein JM. Insulin removal by isolated perfused rat liver. Am J Physiol. 1976;12:171–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

