miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST
- PMID: 24468065
- PMCID: PMC3974149
- DOI: 10.1186/1756-9966-33-12
miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST
Abstract
Background: miRNAs are involved in osteosarcoma (OS) chemoresistance, and TWIST reportedly enhances cisplatin-induced OS cell apoptosis by inhibiting multiple signaling pathways. In this study, we profiled miRNAs differentially expressed in chemoresistant OS, with a focus to identify miRNAs that regulate TWIST expression and OS chemoresistance.
Methods: OS patients who showed <90% tumor necrosis after neochemotherapy were defined as poor responders (chemoresistant), and those who showed ≥90% tumor necrosis were defined as good responders (control). miRNA microarray analysis was carried out with a discovery cohort (n = 12) of age-, sex- and tumor stage-matched chemoresistant and control OS patients.
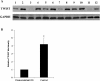
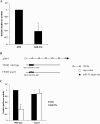
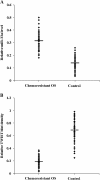
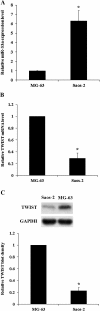
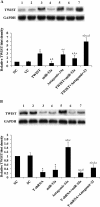
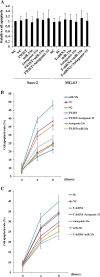
Results: Among the up-regulated miRNAs in chemoresistant OS samples, miR-33a was verified to down-regulate TWIST expression, which was supported by an inverse miRNA-33a/TWIST expression trend in the validation cohort (n = 70), target-sequence-specific inhibition of TWIST-3' untranslated region-luciferase reporter activity by miR-33a, and alteration of TWIST expression by overexpression or inhibition of miR-33a in human OS cell lines. In Saos-2 cells treated with cisplatin, inhibition of miR-33a by antagomir-33a markedly increased cell apoptosis, which was enhanced by overexpression of TWIST. The apoptosis-inducing effect of TWIST overexpression was reversed by overexpression of miR-33a. In MG-63 cells, overexpression of miR-33a significantly decreased cisplatin-induced cell apoptosis, which was enhanced by knockdown of TWIST. Antagomir-33a significantly increased cisplatin-induced cell apoptosis, which was reversed by knockdown of TWIST.
Conclusions: We have demonstrated in this study that miR-33a is up-regulated in chemoresistant OS and that the miR-33a level is negatively correlated with the TWIST protein level in OS. Our in vitro data indicate that miR-33a promotes OS cell resistance to cisplatin by down-regulating TWIST; on the other hand, inhibition of miR-33a by antagomir-33a enhances cisplatin-induced apoptosis in OS cells by up-regulating TWIST expression. The findings suggest that inhibition of miR-33a/TWIST signaling could be a potential new strategy to enhance neoadjuvant chemotherapy for OS.
Figures
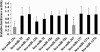
References
-
- Ottaviani G, Jaffe N. In: Pediatric and Adolescent Osteosarcoma. Jaffe N, editor. New York: Springer; 2009. The epidemiology of osteosarcoma; pp. 122–136.
-
- Uribe-Botero G, Russell WO, Sutow WW, Martin RG. Primary osteosarcoma of bone. Clinicopathologic investigation of 243 cases, with necropsy studies in 54. Am J Clin Pathol. 1977;67:427–435. - PubMed
-
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

