Role of gamma carboxylated Glu47 in connexin 26 hemichannel regulation by extracellular Ca²⁺: insight from a local quantum chemistry study
- PMID: 24468086
- PMCID: PMC3969289
- DOI: 10.1016/j.bbrc.2014.01.063
Role of gamma carboxylated Glu47 in connexin 26 hemichannel regulation by extracellular Ca²⁺: insight from a local quantum chemistry study
Abstract
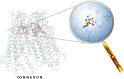
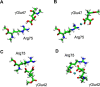
Connexin hemichannels are regulated by several gating mechanisms, some of which depend critically on the extracellular Ca(2+) concentration ([Ca(2+)]e). It is well established that hemichannel activity is inhibited at normal (∼1 mM) [Ca(2+)]e, whereas lowering [Ca(2+)]e to micromolar levels fosters hemichannel opening. Atomic force microscopy imaging shows significant and reversible changes of pore diameter at the extracellular mouth of Cx26 hemichannels exposed to different [Ca(2+)]e, however, the underlying molecular mechanisms are not fully elucidated. Analysis of the crystal structure of connexin 26 (Cx26) gap junction channels, corroborated by molecular dynamics (MD) simulations, suggests that several negatively charged amino acids create a favorable environment for low-affinity Ca(2+) binding within the extracellular vestibule of the Cx26 hemichannel. In particular a highly conserved glutammic acid, found in position 47 in most connexins, is thought to undergo post translational gamma carboxylation (γGlu47), and is thus likely to play an important role in Ca(2+) coordination. γGlu47 may also form salt bridges with two conserved arginines (Arg75 and Arg184 in Cx26), which are considered important in stabilizing the structure of the extracellular region. Using a combination of quantum chemistry methods, we analyzed the interaction between γGlu47, Arg75 and Arg184 in a Cx26 hemichannel model both in the absence and in the presence of Ca(2+). We show that Ca(2+) imparts significant local structural changes and speculate that these modifications may alter the structure of the extracellular loops in Cx26, and may thus account for the mechanism of hemichannel closure in the presence of mM [Ca(2+)]e.
Keywords: Calcium ions; Charcot Marie Tooth disease; Connexin mutations; Deafness; Gating; Hybrid DFT calculations.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
The D50N mutation and syndromic deafness: altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions.J Gen Physiol. 2013 Jul;142(1):3-22. doi: 10.1085/jgp.201310962. J Gen Physiol. 2013. PMID: 23797419 Free PMC article.
-
Insights on the mechanisms of Ca(2+) regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y).J Gen Physiol. 2013 Jul;142(1):23-35. doi: 10.1085/jgp.201210893. J Gen Physiol. 2013. PMID: 23797420 Free PMC article.
-
Mechanism of gating by calcium in connexin hemichannels.Proc Natl Acad Sci U S A. 2016 Dec 6;113(49):E7986-E7995. doi: 10.1073/pnas.1609378113. Epub 2016 Nov 21. Proc Natl Acad Sci U S A. 2016. PMID: 27872296 Free PMC article.
-
Gating of Connexin Channels by transjunctional-voltage: Conformations and models of open and closed states.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):22-39. doi: 10.1016/j.bbamem.2017.04.028. Epub 2017 May 2. Biochim Biophys Acta Biomembr. 2018. PMID: 28476631 Free PMC article. Review.
Cited by
-
Accessing gap-junction channel structure-function relationships through molecular modeling and simulations.BMC Cell Biol. 2017 Jan 17;18(Suppl 1):5. doi: 10.1186/s12860-016-0121-9. BMC Cell Biol. 2017. PMID: 28124624 Free PMC article. Review.
-
Molecular Dynamics Simulation of Permeation Through Connexin Channels.Methods Mol Biol. 2024;2801:45-56. doi: 10.1007/978-1-0716-3842-2_4. Methods Mol Biol. 2024. PMID: 38578412
-
Calcium Regulation of Connexin Hemichannels.Int J Mol Sci. 2024 Jun 15;25(12):6594. doi: 10.3390/ijms25126594. Int J Mol Sci. 2024. PMID: 38928300 Free PMC article. Review.
-
Comparative functional characterization of novel non-syndromic GJB2 gene variant p.Gly45Arg and lethal syndromic variant p.Gly45Glu.PeerJ. 2016 Oct 11;4:e2494. doi: 10.7717/peerj.2494. eCollection 2016. PeerJ. 2016. PMID: 27761313 Free PMC article.
-
Simulations on Simple Models of Connexin Hemichannels Indicate That Ca2+ Blocking Is Not a Pure Electrostatic Effect.Membranes (Basel). 2021 May 20;11(5):372. doi: 10.3390/membranes11050372. Membranes (Basel). 2021. PMID: 34065259 Free PMC article.
References
-
- Sohl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004;62:228–232. - PubMed
-
- Saez J.C., Berthoud V.M., Branes M.C., Martinez A.D., Beyer E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. - PubMed
-
- Pfenniger A., Wohlwend A., Kwak B.R. Mutations in connexin genes and disease. Eur. J. Clin. Invest. 2010;41:103–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

