Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro
- PMID: 24468103
- PMCID: PMC3974148
- DOI: 10.1186/1471-2407-14-43
Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro
Abstract
Background: Thrombocytopenia has been reported to be associated with small size HCCs, and thrombocytosis to be associated with large size HCCs. The aim was to examine the effects of platelets in relation to HCC cell growth.
Methods: The effects of time-expired pooled normal human platelets were examined on human HCC cell line growth and invasion.
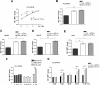
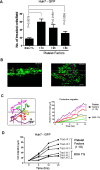
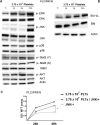
Results: Blood platelet numbers increased with increasing HCC tumor size and portal vein invasion. Platelet extracts enhanced cell growth in 4 human HCC cell lines, as well as cell migration, medium AFP levels and decreased apoptosis. Cell invasion was significantly enhanced, using a Matrigel-coated trans-well membrane and 3D (Real-Time Imaging) invasion assay. Western blots showed that platelets caused enhanced phospho-ERK and phospho-JNK signaling and anti-apoptotic effect with increase of Bcl-xL (anti-apoptotic marker) and decrease of Bid (pro-apoptotic marker) levels. Their growth effects were blocked by a JNK inhibitor.
Conclusions: Platelets stimulated growth and invasion of several HCC cell lines in vitro, suggesting that platelets or platelet growth factors could be a potential pharmacological target.
Figures

Similar articles
-
Platelets are recruited to hepatocellular carcinoma tissues in a CX3CL1-CX3CR1 dependent manner and induce tumour cell apoptosis.Mol Oncol. 2020 Oct;14(10):2546-2559. doi: 10.1002/1878-0261.12783. Epub 2020 Sep 2. Mol Oncol. 2020. PMID: 32799418 Free PMC article.
-
EH-42: A Novel Small Molecule Induces Apoptosis and Inhibits Migration and Invasion of Human Hepatoma Cells through Suppressing STAT3 Signaling Pathway.Curr Cancer Drug Targets. 2019;19(7):583-593. doi: 10.2174/1568009619666181226094814. Curr Cancer Drug Targets. 2019. PMID: 30585547
-
Platelet membrane-derived microparticles may be biomarkers in patients with hepatocellular carcinoma and can promote the invasion and metastasis of hepatoma carcinoma cells.Transfusion. 2023 Oct;63(10):1821-1831. doi: 10.1111/trf.17499. Epub 2023 Sep 8. Transfusion. 2023. PMID: 37680187
-
MicroRNA-294 Promotes Cell Proliferation, Migration and Invasion in SMMC-7721 Hepatoma Carcinoma Cells by Activating the JNK/ERK Signaling Pathway.Am J Med Sci. 2020 Jun;359(6):365-371. doi: 10.1016/j.amjms.2020.03.009. Epub 2020 Mar 9. Am J Med Sci. 2020. PMID: 32498943
-
Modulation of Doxorubicin mediated growth inhibition of hepatocellular carcinoma cells by platelet lysates.Anticancer Agents Med Chem. 2014;14(8):1154-60. doi: 10.2174/1871520614666140604120226. Anticancer Agents Med Chem. 2014. PMID: 24934165
Cited by
-
Platelet lysates in Hepatocellular Carcinoma patients after radiofrequency ablation facilitate tumor proliferation, invasion and vasculogenic mimicry.Int J Med Sci. 2020 Jul 29;17(14):2104-2112. doi: 10.7150/ijms.44405. eCollection 2020. Int J Med Sci. 2020. PMID: 32922171 Free PMC article.
-
Albumin-bilirubin and platelet-albumin-bilirubin grades for hepatitis B-associated hepatocellular carcinoma in Child-Pugh A patients treated with radical surgery: A retrospective observational study.Medicine (Baltimore). 2019 Oct;98(43):e17394. doi: 10.1097/MD.0000000000017394. Medicine (Baltimore). 2019. PMID: 31651841 Free PMC article.
-
A sample model established by S-index predicting overall survival after curative resection of primary hepatocellular carcinoma.Cancer Manag Res. 2019 Jan 14;11:693-703. doi: 10.2147/CMAR.S193593. eCollection 2019. Cancer Manag Res. 2019. PMID: 30679923 Free PMC article.
-
Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma.Liver Cancer. 2019 May;8(3):203-217. doi: 10.1159/000489833. Epub 2018 Jun 22. Liver Cancer. 2019. PMID: 31192156 Free PMC article.
-
Sevoflurane Post-conditioning Protects Primary Rat Cortical Neurons Against Oxygen-Glucose Deprivation/Resuscitation: Roles of Extracellular Signal-Regulated Kinase 1/2 and Bid, Bim, Puma.Neurochem Res. 2015 Aug;40(8):1609-19. doi: 10.1007/s11064-015-1639-5. Epub 2015 Jun 19. Neurochem Res. 2015. PMID: 26088686
References
-
- Ries I. Zurpathologischen Anatomie des Blutes. Arch Anat Physiol Wissensch Med. 1872;39:237–249.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

