Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G
- PMID: 24468108
- PMCID: PMC3909205
- DOI: 10.1186/1742-2094-11-16
Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G
Abstract
Background: Although optic neuritis (ON) is a defining feature of neuromyelitis optica (NMO), appropriate animal models of NMO ON are lacking. Most NMO patients are seropositive for immunoglobulin G autoantibodies (NMO-IgG) against the astrocyte water channel aquaporin-4 (AQP4).
Methods: Several approaches were tested to develop a robust, passive-transfer mouse model of NMO ON, including NMO-IgG and complement delivery by: (i) retrobulbar infusion; (ii) intravitreal injection; (iii) a single intracranial injection near the optic chiasm; and (iv) 3-days continuous intracranial infusion near the optic chiasm.
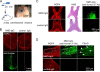
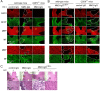
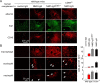
Results: Little ON or retinal pathology was seen using approaches (i) to (iii). Using approach (iv), however, optic nerves showed characteristic NMO pathology, with loss of AQP4 and glial fibrillary acidic protein immunoreactivity, granulocyte and macrophage infiltration, deposition of activated complement, demyelination and axonal injury. Even more extensive pathology was created in mice lacking complement inhibitor protein CD59, or using a genetically modified NMO-IgG with enhanced complement effector function, including significant loss of retinal ganglion cells. In control studies, optic nerve pathology was absent in treated AQP4-deficient mice, or in wild-type mice receiving control (non-NMO) IgG and complement.
Conclusion: Passive transfer of NMO-IgG and complement by continuous infusion near the optic chiasm in mice is sufficient to produce ON with characteristic NMO pathology. The mouse model of NMO ON should be useful in further studies of NMO pathogenesis mechanisms and therapeutics.
Figures


References
-
- Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, Vincent A, Wildemann B. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R01 EY022936/EY/NEI NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

