Mechanism of metabolic stroke and spontaneous cerebral hemorrhage in glutaric aciduria type I
- PMID: 24468193
- PMCID: PMC3940023
- DOI: 10.1186/2051-5960-2-13
Mechanism of metabolic stroke and spontaneous cerebral hemorrhage in glutaric aciduria type I
Abstract
Background: Metabolic stroke is the rapid onset of lasting central neurological deficit associated with decompensation of an underlying metabolic disorder. Glutaric aciduria type I (GA1) is an inherited disorder of lysine and tryptophan metabolism presenting with metabolic stroke in infancy. The clinical presentation includes bilateral striatal necrosis and spontaneous subdural and retinal hemorrhages, which has been frequently misdiagnosed as non-accidental head trauma. The mechanisms underlying metabolic stroke and spontaneous hemorrhage are poorly understood.
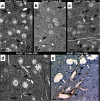
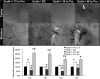
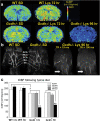
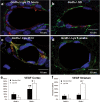
Results: Using a mouse model of GA1, we show that metabolic stroke progresses in the opposite sequence of ischemic stroke, with initial neuronal swelling and vacuole formation leading to cerebral capillary occlusion. Focal regions of cortical followed by striatal capillaries are occluded with shunting to larger non-exchange vessels leading to early filling and dilation of deep cerebral veins. Blood-brain barrier breakdown was associated with displacement of tight-junction protein Occludin.
Conclusion: Together the current findings illuminate the pathophysiology of metabolic stroke and vascular compromise in GA1, which may translate to other neurometabolic disorders presenting with stroke.
Figures




References
-
- Schreiber J, Chapman KA, Summar ML, Ah Mew N, Sutton VR, MacLeod E, Stagni K, Ueda K, Franks J, Island E, Matern D, Pena L, Smith B, Urv T, Venditti C, Chakarapani A, Gropman AL. Neurologic considerations in propionic acidemia. Mol Genet Metab. pp. 10–15. doi: 10.1016/j.ymgme.2011.10.003. - PubMed
-
- Strauss KA, Lazovic J, Wintermark M, Morton DH. Multimodal imaging of striatal degeneration in Amish patients with glutaryl-CoA dehydrogenase deficiency. Brain. 2007;2(Pt 7):1905–1920. doi: 10.1093/brain/awm058. - PubMed
-
- Hoffmann GF, Gibson KM, Trefz FK, Nyhan WL, Bremer HJ, Rating D. Neurological manifestations of organic acid disorders. Eur J Pediatr. 1994;2(7 Suppl 1):S94–S100. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

