Hippocampal granule cell pathology in epilepsy - a possible structural basis for comorbidities of epilepsy?
- PMID: 24468242
- PMCID: PMC4110172
- DOI: 10.1016/j.yebeh.2013.12.022
Hippocampal granule cell pathology in epilepsy - a possible structural basis for comorbidities of epilepsy?
Abstract
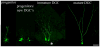
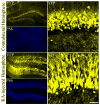
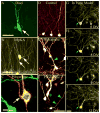
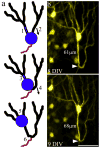
Temporal lobe epilepsy in both animals and humans is characterized by abnormally integrated hippocampal dentate granule cells. Among other abnormalities, these cells make axonal connections with inappropriate targets, grow dendrites in the wrong direction, and migrate to ectopic locations. These changes promote the formation of recurrent excitatory circuits, leading to the appealing hypothesis that these abnormal cells may by epileptogenic. While this hypothesis has been the subject of intense study, less attention has been paid to the possibility that abnormal granule cells in the epileptic brain may also contribute to comorbidities associated with the disease. Epilepsy is associated with a variety of general findings, such as memory disturbances and cognitive dysfunction, and is often comorbid with a number of other conditions, including schizophrenia and autism. Interestingly, recent studies implicate disruption of common genes and gene pathways in all three diseases. Moreover, while neuropsychiatric conditions are associated with changes in a variety of brain regions, granule cell abnormalities in temporal lobe epilepsy appear to be phenocopies of granule cell deficits produced by genetic mouse models of autism and schizophrenia, suggesting that granule cell dysmorphogenesis may be a common factor uniting these seemingly diverse diseases. Disruption of common signaling pathways regulating granule cell neurogenesis may begin to provide mechanistic insight into the cooccurrence of temporal lobe epilepsy and cognitive and behavioral disorders.
Keywords: Autism; Disc1; Epilepsy; Neurogenesis; Reelin; Schizophrenia; mTOR.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interests: The authors declare no competing financial interests
Figures




Similar articles
-
Adult neurogenesis in the intact and epileptic dentate gyrus.Prog Brain Res. 2007;163:529-40. doi: 10.1016/S0079-6123(07)63028-3. Prog Brain Res. 2007. PMID: 17765736 Review.
-
Neuroplasticity in the damaged dentate gyrus of the epileptic brain.Prog Brain Res. 2002;136:319-28. doi: 10.1016/s0079-6123(02)36027-8. Prog Brain Res. 2002. PMID: 12143392 Review.
-
The effect of amygdala kindling on hippocampal neurogenesis coincides with decreased reelin and DISC1 expression in the adult dentate gyrus.Hippocampus. 2010 May;20(5):659-71. doi: 10.1002/hipo.20653. Hippocampus. 2010. PMID: 19499587
-
Disrupted hippocampal network physiology following PTEN deletion from newborn dentate granule cells.Neurobiol Dis. 2016 Dec;96:105-114. doi: 10.1016/j.nbd.2016.09.004. Epub 2016 Sep 3. Neurobiol Dis. 2016. PMID: 27597527 Free PMC article.
-
Altered Synaptic Drive onto Birthdated Dentate Granule Cells in Experimental Temporal Lobe Epilepsy.J Neurosci. 2019 Sep 18;39(38):7604-7614. doi: 10.1523/JNEUROSCI.0654-18.2019. Epub 2019 Jul 3. J Neurosci. 2019. PMID: 31270158 Free PMC article.
Cited by
-
Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder.Nutrients. 2023 Aug 31;15(17):3815. doi: 10.3390/nu15173815. Nutrients. 2023. PMID: 37686847 Free PMC article.
-
Functional disruption of stress modulatory circuits in a model of temporal lobe epilepsy.PLoS One. 2018 May 24;13(5):e0197955. doi: 10.1371/journal.pone.0197955. eCollection 2018. PLoS One. 2018. PMID: 29795651 Free PMC article.
-
Intrauterine inflammation reduces postnatal neurogenesis in the hippocampal subgranular zone and leads to accumulation of hilar ectopic granule cells.Brain Res. 2018 Apr 15;1685:51-59. doi: 10.1016/j.brainres.2018.02.005. Epub 2018 Feb 12. Brain Res. 2018. PMID: 29448014 Free PMC article.
-
Glial source of nitric oxide in epileptogenesis: A target for disease modification in epilepsy.J Neurosci Res. 2019 Nov;97(11):1363-1377. doi: 10.1002/jnr.24205. Epub 2017 Dec 12. J Neurosci Res. 2019. PMID: 29230865 Free PMC article. Review.
-
The FAAH inhibitor URB597 suppresses hippocampal maximal dentate afterdischarges and restores seizure-induced impairment of short and long-term synaptic plasticity.Sci Rep. 2017 Sep 11;7(1):11152. doi: 10.1038/s41598-017-11606-1. Sci Rep. 2017. PMID: 28894217 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

