Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels
- PMID: 24468583
- PMCID: PMC3976757
- DOI: 10.1016/j.actbio.2014.01.020
Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels
Abstract
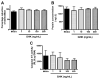
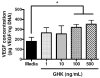
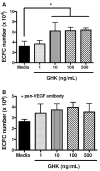
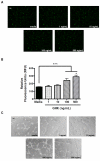
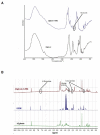
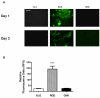
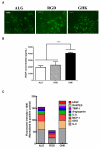
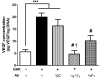
Recombinant proteins and cytokines are under broad preclinical and clinical investigation to promote angiogenesis, but their success is limited by ineffective delivery, lack of long-term stability and excessive cost. Mesenchymal stem/stromal cells (MSC) secrete bioactive trophic factors, and thus, may provide an effective alternative to address these challenges. Glycine-Histidine-Lysine (GHK) is a peptide fragment of osteonectin, a matricellular protein with reported proangiogenic potential. We examined the capacity of GHK to up-regulate secretion of proangiogenic factors from human MSC in culture and when covalently coupled to alginate hydrogels. GHK had no apparent cytotoxic effects on MSC in culture over a wide range of concentrations. We detected a dose-dependent increase in vascular endothelial growth factor (VEGF) concentration in media conditioned by GHK-treated MSC, which increased endothelial cell proliferation, migration and tubule formation. We covalently coupled GHK to alginate using carbodiimide chemistry, and human MSC were entrapped in alginate hydrogels to assess VEGF secretion. Similar to monolayer culture, MSC responded to GHK-modified gels by secreting increased concentrations of VEGF and basic fibroblast growth factor compared to unmodified gels. The pre-treatment of MSC with antibodies to α6 and β1 integrins prior to entrapment in GHK-modified gels abrogated VEGF secretion, suggesting that the proangiogenic response of MSC was integrin-mediated. These data demonstrate that the proangiogenic potential of MSC can be significantly increased by the presentation of GHK with a biodegradable carrier, therefore increasing their clinical potential when used for tissue repair.
Keywords: Alginate; Angiogenesis; GHK; Hydrogel; Vascular endothelial growth factor.
Copyright © 2014 Acta Materialia Inc. All rights reserved.
Figures
Similar articles
-
Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels.Stem Cells Transl Med. 2016 Jun;5(6):773-81. doi: 10.5966/sctm.2015-0211. Epub 2016 Apr 7. Stem Cells Transl Med. 2016. PMID: 27057004 Free PMC article.
-
Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells.Tissue Eng Part A. 2013 Jun;19(11-12):1424-32. doi: 10.1089/ten.TEA.2012.0581. Epub 2013 Feb 26. Tissue Eng Part A. 2013. PMID: 23327676 Free PMC article.
-
Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold.Tissue Eng Part A. 2014 Feb;20(3-4):611-21. doi: 10.1089/ten.TEA.2013.0229. Epub 2013 Nov 6. Tissue Eng Part A. 2014. PMID: 24070211 Free PMC article.
-
Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments.Acta Biomater. 2011 Apr;7(4):1674-82. doi: 10.1016/j.actbio.2010.12.029. Epub 2010 Dec 28. Acta Biomater. 2011. PMID: 21193068
-
The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: implications for cognitive health.Oxid Med Cell Longev. 2012;2012:324832. doi: 10.1155/2012/324832. Epub 2012 May 10. Oxid Med Cell Longev. 2012. PMID: 22666519 Free PMC article. Review.
Cited by
-
Bone Morphogenetic Protein-2 Promotes Human Mesenchymal Stem Cell Survival and Resultant Bone Formation When Entrapped in Photocrosslinked Alginate Hydrogels.Adv Healthc Mater. 2016 Oct;5(19):2501-2509. doi: 10.1002/adhm.201600461. Epub 2016 Sep 1. Adv Healthc Mater. 2016. PMID: 27581621 Free PMC article.
-
Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies.Stem Cell Res Ther. 2016 Sep 9;7(1):131. doi: 10.1186/s13287-016-0394-0. Stem Cell Res Ther. 2016. PMID: 27612948 Free PMC article. Review.
-
Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo.J Control Release. 2015 Nov 10;217:191-201. doi: 10.1016/j.jconrel.2015.09.005. Epub 2015 Sep 11. J Control Release. 2015. PMID: 26365781 Free PMC article.
-
Electrospun fibers enhanced the paracrine signaling of mesenchymal stem cells for cartilage regeneration.Stem Cell Res Ther. 2021 Feb 3;12(1):100. doi: 10.1186/s13287-021-02137-8. Stem Cell Res Ther. 2021. PMID: 33536060 Free PMC article.
-
GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration.Biomed Res Int. 2015;2015:648108. doi: 10.1155/2015/648108. Epub 2015 Jul 7. Biomed Res Int. 2015. PMID: 26236730 Free PMC article. Review.
References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. - PubMed
-
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–66. - PubMed
-
- Ribatti D, Baiguera S. Phase II angiogenesis stimulators. Expert Opin Investig Drugs. 2013;22:1157–66. - PubMed
-
- Mitsos S, Katsanos K, Koletsis E, Kagadis GC, Anastasiou N, Diamantopoulos A, et al. Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials. Angiogenesis. 2012;15:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

