Class D β-lactamases: are they all carbapenemases?
- PMID: 24468778
- PMCID: PMC4023754
- DOI: 10.1128/AAC.02522-13
Class D β-lactamases: are they all carbapenemases?
Abstract
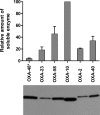
Carbapenem-hydrolyzing class D β-lactamases (CHDLs) are enzymes of the utmost clinical importance due to their ability to produce resistance to carbapenems, the antibiotics of last resort for the treatment of various life-threatening infections. The vast majority of these enzymes have been identified in Acinetobacter spp., notably in Acinetobacter baumannii. The OXA-2 and OXA-10 enzymes predominantly occur in Pseudomonas aeruginosa and are currently classified as narrow-spectrum class D β-lactamases. Here we demonstrate that when OXA-2 and OXA-10 are expressed in Escherichia coli strain JM83, they produce a narrow-spectrum antibiotic resistance pattern. When the enzymes are expressed in A. baumannii ATCC 17978, however, they behave as extended-spectrum β-lactamases and confer resistance to carbapenem antibiotics. Kinetic studies of OXA-2 and OXA-10 with four carbapenems have demonstrated that their catalytic efficiencies with these antibiotics are in the same range as those of some recognized class D carbapenemases. These results are in disagreement with the classification of the OXA-2 and OXA-10 enzymes as narrow-spectrum β-lactamases, and they suggest that other class D enzymes that are currently regarded as noncarbapenemases may in fact be CHDLs.
Figures
Similar articles
-
WCK 4234, a novel diazabicyclooctane potentiating carbapenems against Enterobacteriaceae, Pseudomonas and Acinetobacter with class A, C and D β-lactamases.J Antimicrob Chemother. 2017 Jun 1;72(6):1688-1695. doi: 10.1093/jac/dkx035. J Antimicrob Chemother. 2017. PMID: 28333319
-
Activity of the β-Lactamase Inhibitor LN-1-255 against Carbapenem-Hydrolyzing Class D β-Lactamases from Acinetobacter baumannii.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01172-17. doi: 10.1128/AAC.01172-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28807908 Free PMC article.
-
Structural Insights into the Mechanism of Carbapenemase Activity of the OXA-48 β-Lactamase.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e01202-19. doi: 10.1128/AAC.01202-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31358584 Free PMC article.
-
Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology.Clin Microbiol Infect. 2006 Sep;12(9):826-36. doi: 10.1111/j.1469-0691.2006.01456.x. Clin Microbiol Infect. 2006. PMID: 16882287 Review.
-
OXA-type carbapenemases in Acinetobacter baumannii in South America.J Infect Dev Ctries. 2012 Apr 13;6(4):311-6. doi: 10.3855/jidc.2310. J Infect Dev Ctries. 2012. PMID: 22505439 Review.
Cited by
-
Metallo-β-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design.Chem Rev. 2021 Jul 14;121(13):7957-8094. doi: 10.1021/acs.chemrev.1c00138. Epub 2021 Jun 15. Chem Rev. 2021. PMID: 34129337 Free PMC article. Review.
-
First Report of bla OXA-677 with Enhanced Meropenem-Hydrolyzing Ability in Pseudomonas aeruginosa in China.Infect Drug Resist. 2021 Dec 31;14:5725-5733. doi: 10.2147/IDR.S340662. eCollection 2021. Infect Drug Resist. 2021. PMID: 35002263 Free PMC article.
-
Antibacterial Spectrum of a Tetrazole-Based Reversible Inhibitor of Serine β-Lactamases.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e02563-17. doi: 10.1128/AAC.02563-17. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29844038 Free PMC article.
-
The C5α-Methyl-Substituted Carbapenem NA-1-157 Exhibits Potent Activity against Klebsiella spp. Isolates Producing OXA-48-Type Carbapenemases.ACS Infect Dis. 2023 May 12;9(5):1123-1136. doi: 10.1021/acsinfecdis.3c00059. Epub 2023 May 2. ACS Infect Dis. 2023. PMID: 37130087 Free PMC article.
-
Past and Present Perspectives on β-Lactamases.Antimicrob Agents Chemother. 2018 Sep 24;62(10):e01076-18. doi: 10.1128/AAC.01076-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30061284 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

