Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow
- PMID: 24469165
- PMCID: PMC4066306
- DOI: 10.1097/01.prs.0000436840.69752.7e
Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow
Abstract
Background: Although lymph node transplantation has been shown to improve lymphatic function, the mechanisms regulating lymphatic vessel reconnection and functional status of lymph nodes remains poorly understood.
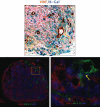
Methods: The authors developed and used LacZ lymphatic reporter mice to examine the lineage of lymphatic vessels infiltrating transferred lymph nodes. In addition, the authors analyzed lymphatic function, expression of vascular endothelial growth factor (VEGF)-C, maintenance of T- and B-cell zone, and anatomical localization of lymphatics and high endothelial venules.
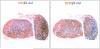
Results: Reporter mice were specific and highly sensitive in identifying lymphatic vessels. Lymph node transfer was associated with rapid return of lymphatic function and clearance of technetium-99 secondary to a massive infiltration of recipient mouse lymphatics and putative connections to donor lymphatics. T- and B-cell populations in the lymph node were maintained. These changes correlated with marked increases in the expression of VEGF-C in the perinodal fat and infiltrating lymphatics. Newly formed lymphatic channels in transferred lymph nodes were in close anatomical proximity to high endothelial venules.
Conclusions: Transferred lymph nodes have rapid infiltration of functional host lymphatic vessels and maintain T- and B-cell populations. This process correlates with increased endogenous expression of VEGF-C in the perinodal fat and infiltrating lymphatics. Anatomical proximity of newly formed lymphatics and high endothelial venules supports the hypothesis that lymph node transfer can improve lymphedema by exchanges with the systemic circulation.
Figures



References
-
- Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. - PubMed
-
- Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131:1286–1298. - PubMed
-
- Tobbia D, Semple J, Baker A, et al. Experimental assessment of autologous lymph node transplantation as treatment of postsurgical lymphedema. Plast Reconstr Surg. 2009;124:777–786. - PubMed
-
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg. 2012;255:468–473. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

