Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes
- PMID: 24469401
- PMCID: PMC3993574
- DOI: 10.1128/MCB.01176-13
Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes
Abstract
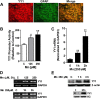
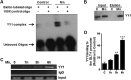
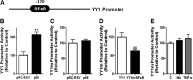
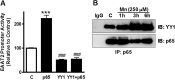
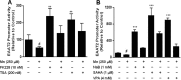
Impairment of astrocytic glutamate transporter (GLT-1; EAAT2) function is associated with multiple neurodegenerative diseases, including Parkinson's disease (PD) and manganism, the latter being induced by chronic exposure to high levels of manganese (Mn). Mn decreases EAAT2 promoter activity and mRNA and protein levels, but the molecular mechanism of Mn-induced EAAT2 repression at the transcriptional level has yet to be elucidated. We reveal that transcription factor Yin Yang 1 (YY1) is critical in repressing EAAT2 and mediates the effects of negative regulators, such as Mn and tumor necrosis factor alpha (TNF-α), on EAAT2. YY1 overexpression in astrocytes reduced EAAT2 promoter activity, while YY1 knockdown or mutation of the YY1 consensus site of the EAAT2 promoter increased its promoter activity and attenuated the Mn-induced repression of EAAT2. Mn increased YY1 promoter activity and mRNA and protein levels via NF-κB activation. This led to increased YY1 binding to the EAAT2 promoter region. Epigenetically, histone deacetylase (HDAC) classes I and II served as corepressors of YY1, and, accordingly, HDAC inhibitors increased EAAT2 promoter activity and reversed the Mn-induced repression of EAAT2 promoter activity. Taken together, our findings suggest that YY1, with HDACs as corepressors, is a critical negative transcriptional regulator of EAAT2 and mediates Mn-induced EAAT2 repression.
Figures

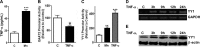
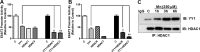
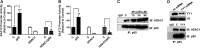

Similar articles
-
Manganese phosphorylates Yin Yang 1 at serine residues to repress EAAT2 in human H4 astrocytes.Toxicol Lett. 2022 Feb 1;355:41-46. doi: 10.1016/j.toxlet.2021.11.007. Epub 2021 Nov 17. Toxicol Lett. 2022. PMID: 34800614 Free PMC article.
-
Manganese-induced reactive oxygen species activate IκB kinase to upregulate YY1 and impair glutamate transporter EAAT2 function in human astrocytes in vitro.Neurotoxicology. 2021 Sep;86:94-103. doi: 10.1016/j.neuro.2021.07.004. Epub 2021 Jul 24. Neurotoxicology. 2021. PMID: 34310962 Free PMC article.
-
Transcriptional Regulation of the Astrocytic Excitatory Amino Acid Transporter 1 (EAAT1) via NF-κB and Yin Yang 1 (YY1).J Biol Chem. 2015 Sep 25;290(39):23725-37. doi: 10.1074/jbc.M115.649327. Epub 2015 Aug 12. J Biol Chem. 2015. PMID: 26269591 Free PMC article.
-
Genetic dys-regulation of astrocytic glutamate transporter EAAT2 and its implications in neurological disorders and manganese toxicity.Neurochem Res. 2015 Feb;40(2):380-8. doi: 10.1007/s11064-014-1391-2. Epub 2014 Jul 27. Neurochem Res. 2015. PMID: 25064045 Free PMC article. Review.
-
Role of transcription factor yin yang 1 in manganese-induced reduction of astrocytic glutamate transporters: Putative mechanism for manganese-induced neurotoxicity.Neurochem Int. 2015 Sep;88:53-9. doi: 10.1016/j.neuint.2014.08.002. Epub 2014 Aug 13. Neurochem Int. 2015. PMID: 25128239 Free PMC article. Review.
Cited by
-
Transcriptional Regulation of Glutamate Transporters: From Extracellular Signals to Transcription Factors.Adv Pharmacol. 2016;76:103-45. doi: 10.1016/bs.apha.2016.01.004. Epub 2016 Mar 24. Adv Pharmacol. 2016. PMID: 27288076 Free PMC article. Review.
-
17β-estradiol and tamoxifen protect mice from manganese-induced dopaminergic neurotoxicity.Neurotoxicology. 2018 Mar;65:280-288. doi: 10.1016/j.neuro.2017.11.008. Epub 2017 Nov 26. Neurotoxicology. 2018. PMID: 29183790 Free PMC article.
-
Mutant GGGGCC RNA prevents YY1 from binding to Fuzzy promoter which stimulates Wnt/β-catenin pathway in C9ALS/FTD.Nat Commun. 2023 Dec 18;14(1):8420. doi: 10.1038/s41467-023-44215-w. Nat Commun. 2023. PMID: 38110419 Free PMC article.
-
Cellular Pathogenesis of Hepatic Encephalopathy: An Update.Biomolecules. 2023 Feb 19;13(2):396. doi: 10.3390/biom13020396. Biomolecules. 2023. PMID: 36830765 Free PMC article. Review.
-
Critical Involvement of Glial Cells in Manganese Neurotoxicity.Biomed Res Int. 2021 Oct 6;2021:1596185. doi: 10.1155/2021/1596185. eCollection 2021. Biomed Res Int. 2021. PMID: 34660781 Free PMC article. Review.
References
-
- Meldrum BS. 2000. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 130(4S Suppl):1007S–1015S - PubMed
-
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. 2011. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J. Cell. Physiol. 226:2484–2493. 10.1002/jcp.22609 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
