Structural insights into neonatal Fc receptor-based recycling mechanisms
- PMID: 24469444
- PMCID: PMC3953293
- DOI: 10.1074/jbc.M113.537563
Structural insights into neonatal Fc receptor-based recycling mechanisms
Abstract
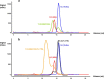
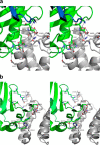
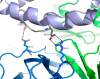
We report the three-dimensional structure of human neonatal Fc receptor (FcRn) bound concurrently to its two known ligands. More particularly, we solved the crystal structure of the complex between human FcRn, wild-type human serum albumin (HSA), and a human Fc engineered for improved pharmacokinetics properties (Fc-YTE). The crystal structure of human FcRn bound to wild-type HSA alone is also presented. HSA domain III exhibits an extensive interface of contact with FcRn, whereas domain I plays a lesser role. A molecular explanation for the HSA recycling mechanism is provided with the identification of FcRn His(161) as the only potential direct contributor to the corresponding pH-dependent process. At last, this study also allows an accurate structural definition of residues considered for decades as important to the human IgG/FcRn interaction and reveals Fc His(310) as a significant contributor to pH-dependent binding. Finally, we explain various structural mechanisms by which several Fc mutations (including YTE) result in increased human IgG binding to FcRn. Our study provides an unprecedented relevant understanding of the molecular basis of human Fc interaction with human FcRn.
Keywords: Albumin; Antibodies; Crystal Structure; FC Receptors; Protein Complexes.
Figures




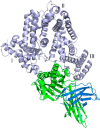


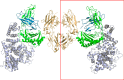


References
-
- Simister N. E., Mostov K. E. (1989) An Fc receptor structurally related to MHC class I antigens. Nature 337, 184–187 - PubMed
-
- Burmeister W. P., Gastinel L. N., Simister N. E., Blum M. L., Bjorkman P. J. (1994) Crystal structure at 2.2-Å resolution of the MHC-related neonatal Fc receptor. Nature 372, 336–343 - PubMed
-
- Burmeister W. P., Huber A. H., Bjorkman P. J. (1994) Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 372, 379–383 - PubMed
-
- Raghavan M., Bonagura V. R., Morrison S. L., Bjorkman P. J. (1995) Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 34, 14649–14657 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

