The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation
- PMID: 24469458
- PMCID: PMC3945327
- DOI: 10.1074/jbc.M113.519256
The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation
Abstract
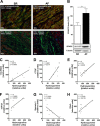
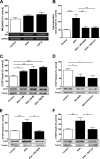
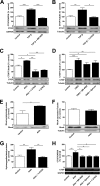
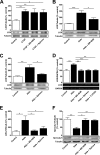
We studied the role of the mineralocorticoid receptor (MR) in the signaling that promotes atrial fibrosis. Left atrial myocardium of patients with atrial fibrillation (AF) exhibited 4-fold increased hydroxyproline content compared with patients in sinus rhythm. Expression of MR was similar, as was 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which also increased. 11β-HSD2 converts cortisol to receptor-inactive metabolites allowing aldosterone occupancy of MR. 11β-HSD2 was up-regulated by arrhythmic pacing in cultured cardiomyocytes and in a mouse model of spontaneous AF (RacET). In cardiomyocytes, aldosterone induced connective tissue growth factor (CTGF) in the absence but not in the presence of cortisol. Hydroxyproline expression was increased in cardiac fibroblasts exposed to conditioned medium from aldosterone-treated cardiomyocytes but not from cardiomyocytes treated with both cortisol and aldosterone. Aldosterone increased connective tissue growth factor and hydroxyproline expression in cardiac fibroblasts, which were prevented by BR-4628, a dihydropyridine-derived selective MR antagonist, and by spironolactone. Aldosterone activated RhoA GTPase. Rho kinase inhibition by Y-27632 prevented CTGF and hydroxyproline, whereas the RhoA activator CN03 increased CTGF expression. Aldosterone and CTGF increased lysyl oxidase, and aldosterone enhanced miR-21 expression. MR antagonists reduced the aldosterone but not the CTGF effect. In conclusion, MR signaling promoted fibrotic remodeling. Increased expression of 11β-HSD2 during AF leads to up-regulation of collagen and pro-fibrotic mediators by aldosterone, specifically RhoA activity as well as CTGF, lysyl oxidase, and microRNA-21 expression. The MR antagonists BR-4628 and spironolactone prevent these alterations. MR inhibition may, therefore, represent a potential pharmacologic target for the prevention of fibrotic remodeling of the atrial myocardium.
Keywords: CTGF; Fibrosis; Heart; Lysyl Oxidase; MicroRNA; Mineralocorticoid Receptor.
Figures


References
-
- Lip G. Y., Tse H. F., Lane D. A. (2012) Atrial fibrillation. Lancet 379, 648–661 - PubMed
-
- Burstein B., Nattel S. (2008) Atrial fibrosis. Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 51, 802–809 - PubMed
-
- Nattel S., Burstein B., Dobrev D. (2008) Atrial remodeling and atrial fibrillation. Mechanisms and implications. Circ. Arrhythm Electrophysiol. 1, 62–73 - PubMed
-
- Dobrev D., Carlsson L., Nattel S. (2012) Novel molecular targets for atrial fibrillation therapy. Nat. Rev. Drug. Discov. 11, 275–291 - PubMed
-
- Zhou G., Kandala J. C., Tyagi S. C., Katwa L. C., Weber K. T. (1996) Effects of angiotensin II and aldosterone on collagen gene expression and protein turnover in cardiac fibroblasts. Mol. Cell. Biochem. 154, 171–178 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

