Nitration of tyrosine 247 inhibits protein kinase G-1α activity by attenuating cyclic guanosine monophosphate binding
- PMID: 24469460
- PMCID: PMC3953305
- DOI: 10.1074/jbc.M113.534313
Nitration of tyrosine 247 inhibits protein kinase G-1α activity by attenuating cyclic guanosine monophosphate binding
Abstract
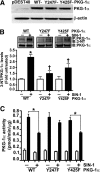
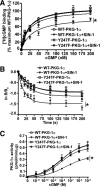
The cGMP-dependent protein kinase G-1α (PKG-1α) is a downstream mediator of nitric oxide and natriuretic peptide signaling. Alterations in this pathway play a key role in the pathogenesis and progression of vascular diseases associated with increased vascular tone and thickness, such as pulmonary hypertension. Previous studies have shown that tyrosine nitration attenuates PKG-1α activity. However, little is known about the mechanisms involved in this event. Utilizing mass spectrometry, we found that PKG-1α is susceptible to nitration at tyrosine 247 and 425. Tyrosine to phenylalanine mutants, Y247F- and Y425F-PKG-1α, were both less susceptible to nitration than WT PKG-1α, but only Y247F-PKG-1α exhibited preserved activity, suggesting that the nitration of Tyr(247) is critical in attenuating PKG-1α activity. The overexpression of WT- or Y247F-PKG-1α decreased the proliferation of pulmonary artery smooth muscle cells (SMC), increased the expression of SMC contractile markers, and decreased the expression of proliferative markers. Nitrosative stress induced a switch from a contractile to a synthetic phenotype in cells expressing WT- but not Y247F-PKG-1α. An antibody generated against 3-NT-Y247 identified increased levels of nitrated PKG-1α in humans with pulmonary hypertension. Finally, to gain a more mechanistic understanding of how nitration attenuates PKG activity, we developed a homology model of PKG-1α. This model predicted that the nitration of Tyr(247) would decrease the affinity of PKG-1α for cGMP, which we confirmed using a [(3)H]cGMP binding assay. Our study shows that the nitration of Tyr(247) and the attenuation of cGMP binding is an important mechanism regulating in PKG-1α activity and SMC proliferation/differentiation.
Keywords: Cyclic GMP (cGMP); Enzyme Catalysis; Mass Spectrometry (MS); Molecular Modeling; Peroxynitrite; Protein Kinase G (PKG).
Figures





References
-
- Aggarwal S., Gross C. M., Kumar S., Datar S., Oishi P., Kalkan G., Schreiber C., Fratz S., Fineman J. R., Black S. M. (2011) Attenuated vasodilatation in lambs with endogenous and exogenous activation of cGMP signaling. Role of protein kinase G nitration. J. Cell. Physiol. 226, 3104–3113 - PMC - PubMed
-
- Negash S., Gao Y., Zhou W., Liu J., Chinta S., Raj J. U. (2007) Regulation of cGMP-dependent protein kinase-mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L1012–L1020 - PubMed
-
- Zhao Y. Y., Zhao Y. D., Mirza M. K., Huang J. H., Potula H. H., Vogel S. M., Brovkovych V., Yuan J. X., Wharton J., Malik A. B. (2009) Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J. Clin. Invest. 119, 2009–2018 - PMC - PubMed
-
- Garbers D. L. (1992) Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell 71, 1–4 - PubMed
-
- Feil S., Zimmermann P., Knorn A., Brummer S., Schlossmann J., Hofmann F., Feil R. (2005) Distribution of cGMP-dependent protein kinase type I and its isoforms in the mouse brain and retina. Neuroscience 135, 863–868 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

