HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine
- PMID: 24469503
- PMCID: PMC3939461
- DOI: 10.3390/v6020371
HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine
Abstract
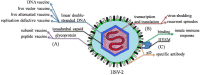
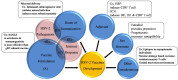
Herpes simplex virus type 2 (HSV-2), a globally sexually transmitted virus, and also one of the main causes of genital ulcer diseases, increases susceptibility to HIV-1. Effective vaccines to prevent HSV-2 infection are not yet available, but are currently being developed. To facilitate this process, the latest progress in development of these vaccines is reviewed in this paper. A summary of the most promising HSV-2 vaccines tested in animals in the last five years is presented, including the main factors, and new ideas for developing an effective vaccine from animal experiments and human clinical trials. Experimental results indicate that future HSV-2 vaccines may depend on a strategy that targets mucosal immunity. Furthermore, estradiol, which increases the effectiveness of vaccines, may be considered as an adjuvant. Therefore, this review is expected to provide possible strategies for development of future HSV-2 vaccines.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

