TBC1D24 regulates neuronal migration and maturation through modulation of the ARF6-dependent pathway
- PMID: 24469796
- PMCID: PMC3926028
- DOI: 10.1073/pnas.1316294111
TBC1D24 regulates neuronal migration and maturation through modulation of the ARF6-dependent pathway
Abstract
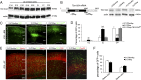
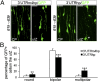
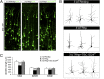
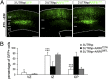
Alterations in the formation of brain networks are associated with several neurodevelopmental disorders. Mutations in TBC1 domain family member 24 (TBC1D24) are responsible for syndromes that combine cortical malformations, intellectual disability, and epilepsy, but the function of TBC1D24 in the brain remains unknown. We report here that in utero TBC1D24 knockdown in the rat developing neocortex affects the multipolar-bipolar transition of neurons leading to delayed radial migration. Furthermore, we find that TBC1D24-knockdown neurons display an abnormal maturation and retain immature morphofunctional properties. TBC1D24 interacts with ADP ribosylation factor (ARF)6, a small GTPase crucial for membrane trafficking. We show that in vivo, overexpression of the dominant-negative form of ARF6 rescues the neuronal migration and dendritic outgrowth defects induced by TBC1D24 knockdown, suggesting that TBC1D24 prevents ARF6 activation. Overall, our findings demonstrate an essential role of TBC1D24 in neuronal migration and maturation and highlight the physiological relevance of the ARF6-dependent membrane-trafficking pathway in brain development.
Keywords: RNA interference; dendritogenesis; epileptic encephalopathies; gene; synaptogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ayala R, Shu T, Tsai LH. Trekking across the brain: The journey of neuronal migration. Cell. 2007;128(1):29–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

