Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass
- PMID: 24469798
- PMCID: PMC3926031
- DOI: 10.1073/pnas.1324019111
Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass
Abstract
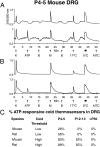
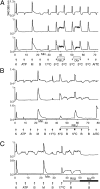
Change is intrinsic to nervous systems; change is required for learning and conditioning and occurs with disease progression, normal development, and aging. To better understand mammalian nervous systems and effectively treat nervous-system disorders, it is essential to track changes in relevant individual neurons. A critical challenge is to identify and characterize the specific cell types involved and the molecular-level changes that occur in each. Using an experimental strategy called constellation pharmacology, we demonstrate that we can define a specific somatosensory neuronal subclass, cold thermosensors, across different species and track changes in these neurons as a function of development. Cold thermosensors are uniformly responsive to menthol and innocuous cool temperature (17 °C), indicating that they express TRPM8 channels. A subset of cold thermosensors expressed α7 nicotinic acetylcholine receptors (nAChRs) but not other nAChR subtypes. Differences in temperature threshold of cold thermosensors correlated with functional expression of voltage-gated K channels Kv1.1/1.2: Relatively higher expression of KV1.1/1.2 channels resulted in a higher threshold response to cold temperature. Other signaling components varied during development and between species. In cold thermosensors of neonatal mice and rats, ATP receptors were functionally expressed, but the expression disappeared with development. This developmental change occurred earlier in low-threshold than high-threshold cold thermosensors. Most rat cold thermosensors expressed TRPA1 channels, whereas mouse cold thermosensors did not. The broad implications of this study are that it is now feasible to track changes in receptor and ion-channel expression in individual neuronal subclasses as a function of development, learning, disease, or aging.
Keywords: DRG; KV1.2; calcium imaging; purinergic receptor; trigeminal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Investigating neuronal cell types through comparative cellular physiology.Temperature (Austin). 2014 Jun 24;1(1):22-3. doi: 10.4161/temp.29540. eCollection 2014 Apr-Jun. Temperature (Austin). 2014. PMID: 27580692 Free PMC article.
References
-
- Reiner A, Isacoff EY. The Brain Prize 2013: The optogenetics revolution. Trends Neurosci. 2013;36(10):557–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

