Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers
- PMID: 24469818
- PMCID: PMC3926063
- DOI: 10.1073/pnas.1317431111
Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers
Abstract
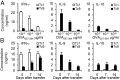
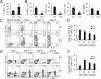
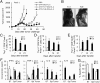
Because cytokine-priming signals direct CD8(+) T cells to acquire unique profiles that affect their ability to mediate specific immune responses, here we generated IL-9-skewed CD8(+) T (Tc9) cells by priming with Th9-polarized condition. Compared with type-I CD8(+) cytotoxic T (Tc1) cells, Tc9 secreted different cytokines and were less cytolytic in vitro but surprisingly elicited greater antitumor responses against advanced tumors in OT-I/B16-OVA and Pmel-1/B16 melanoma models. After adoptive transfer, Tc9 cells persisted longer and differentiated into IFN-γ- and granzyme-B (GrzB)-producing cytolytic Tc1-like effector cells. Phenotypic analysis revealed that adoptively transferred Tc9 cells secreted IL-2 and were KLRG-1(low) and IL-7Rα(high), suggesting that they acquired a signature of "younger" phenotype or became long-term lived cells with capacity of self-renewal. Our results also revealed that Tc9-mediated therapeutic effect critically depended on IL-9 production in vivo. These findings have clinical implications for the improvement of CD8(+) T-cell-based adoptive immunotherapy of cancers.
Keywords: T-cell lineage plasticity; adoptive cell therapy; less-exhausted T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses.J Immunol. 2004 Feb 1;172(3):1380-90. doi: 10.4049/jimmunol.172.3.1380. J Immunol. 2004. PMID: 14734713
-
Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms.J Immunol. 2013 Feb 15;190(4):1873-81. doi: 10.4049/jimmunol.1201989. Epub 2013 Jan 11. J Immunol. 2013. PMID: 23315072 Free PMC article.
-
Cholesterol negatively regulates IL-9-producing CD8+ T cell differentiation and antitumor activity.J Exp Med. 2018 Jun 4;215(6):1555-1569. doi: 10.1084/jem.20171576. Epub 2018 May 9. J Exp Med. 2018. PMID: 29743292 Free PMC article.
-
Heterogeneity in the differentiation and function of CD8⁺ T cells.Arch Immunol Ther Exp (Warsz). 2014 Dec;62(6):449-58. doi: 10.1007/s00005-014-0293-y. Epub 2014 May 31. Arch Immunol Ther Exp (Warsz). 2014. PMID: 24879097 Review.
-
Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy.Immunol Rev. 2014 Jan;257(1):264-276. doi: 10.1111/imr.12135. Immunol Rev. 2014. PMID: 24329803 Free PMC article. Review.
Cited by
-
The Role of Interleukin-9 in Cancer.Pathol Oncol Res. 2020 Oct;26(4):2017-2022. doi: 10.1007/s12253-019-00665-6. Epub 2019 Apr 23. Pathol Oncol Res. 2020. PMID: 31016637 Review.
-
Less cholesterol means better tumor killing for cytotoxic T9 cells.J Exp Med. 2018 Jun 4;215(6):1505-1506. doi: 10.1084/jem.20180852. Epub 2018 May 15. J Exp Med. 2018. PMID: 29764914 Free PMC article.
-
TGF-β in T Cell Biology: Implications for Cancer Immunotherapy.Cancers (Basel). 2018 Jun 11;10(6):194. doi: 10.3390/cancers10060194. Cancers (Basel). 2018. PMID: 29891791 Free PMC article. Review.
-
Expression of a Chimeric Antigen Receptor in Multiple Leukocyte Lineages in Transgenic Mice.PLoS One. 2015 Oct 27;10(10):e0140543. doi: 10.1371/journal.pone.0140543. eCollection 2015. PLoS One. 2015. PMID: 26505904 Free PMC article.
-
Dual Face of Vγ9Vδ2-T Cells in Tumor Immunology: Anti- versus Pro-Tumoral Activities.Front Immunol. 2017 Aug 28;8:1041. doi: 10.3389/fimmu.2017.01041. eCollection 2017. Front Immunol. 2017. PMID: 28894450 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

