CD28 and CD3 have complementary roles in T-cell traction forces
- PMID: 24469820
- PMCID: PMC3926067
- DOI: 10.1073/pnas.1315606111
CD28 and CD3 have complementary roles in T-cell traction forces
Abstract
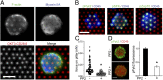
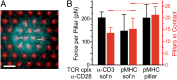
Mechanical forces have key roles in regulating activation of T cells and coordination of the adaptive immune response. A recent example is the ability of T cells to sense the rigidity of an underlying substrate through the T-cell receptor (TCR) coreceptor CD3 and CD28, a costimulation signal essential for cell activation. In this report, we show that these two receptor systems provide complementary functions in regulating the cellular forces needed to test the mechanical properties of the extracellular environment. Traction force microscopy was carried out on primary human cells interacting with micrometer-scale elastomer pillar arrays presenting activation antibodies to CD3 and/or CD28. T cells generated traction forces of 100 pN on arrays with both antibodies. By providing one antibody or the other in solution instead of on the pillars, we show that force generation is associated with CD3 and the TCR complex. Engagement of CD28 increases traction forces associated with CD3 through the signaling pathway involving PI3K, rather than providing additional coupling between the cell and surface. Force generation is concentrated to the cell periphery and associated with molecular complexes containing phosphorylated Pyk2, suggesting that T cells use processes that share features with integrin signaling in force generation. Finally, the ability of T cells to apply forces through the TCR itself, rather than the CD3 coreceptor, was tested. Mouse cells expressing the 5C.C7 TCR exerted traction forces on pillars presenting peptide-loaded MHCs that were similar to those with α-CD3, suggesting that forces are applied to antigen-presenting cells during activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

