Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring
- PMID: 24469827
- PMCID: PMC3926084
- DOI: 10.1073/pnas.1305609111
Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring
Abstract
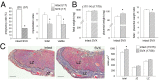
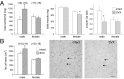
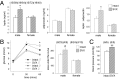
Paternal characteristics and exposures influence physiology and disease risks in progeny, but the mechanisms are mostly unknown. Seminal fluid, which affects female reproductive tract gene expression as well as sperm survival and integrity, provides one potential pathway. We evaluated in mice the consequences for offspring of ablating the plasma fraction of seminal fluid by surgical excision of the seminal vesicle gland. Conception was substantially impaired and, when pregnancy did occur, placental hypertrophy was evident in late gestation. After birth, the growth trajectory and metabolic parameters of progeny were altered, most profoundly in males, which exhibited obesity, distorted metabolic hormones, reduced glucose tolerance, and hypertension. Altered offspring phenotype was partly attributable to sperm damage and partly to an effect of seminal fluid deficiency on the female tract, because increased adiposity was also evident in adult male progeny when normal two-cell embryos were transferred to females mated with seminal vesicle-excised males. Moreover, embryos developed in female tracts not exposed to seminal plasma were abnormal from the early cleavage stages, but culture in vitro partly alleviated this. Absence of seminal plasma was accompanied by down-regulation of the embryotrophic factors Lif, Csf2, Il6, and Egf and up-regulation of the apoptosis-inducing factor Trail in the oviduct. These findings show that paternal seminal fluid composition affects the growth and health of male offspring, and reveal that its impact on the periconception environment involves not only sperm protection but also indirect effects on preimplantation embryos via oviduct expression of embryotrophic cytokines.
Keywords: embryo development; fertility; growth factors; metabolic disorder; programming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
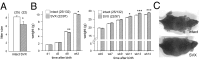
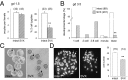
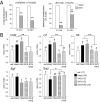
References
-
- Poiani A. Complexity of seminal fluid: A review. Behav Ecol Sociobiol. 2006;60(3):289–310.
-
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322(1):43–52. - PubMed
-
- Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. - PubMed
-
- Tremellen KP, Seamark RF, Robertson SA. Seminal TGFbeta1 stimulates GM-CSF production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58(5):1217–1225. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

