Kindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesis
- PMID: 24469992
- PMCID: PMC3986835
- DOI: 10.1096/fj.13-244004
Kindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesis
Abstract
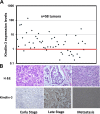
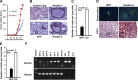
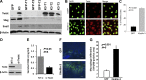
The FERM domain containing protein Kindlin-3 has been recognized as a major regulator of integrin function in hematopoietic cells, but its role in neoplasia is totally unknown. We have examined the relationship between Kindlin-3 and breast cancer in mouse models and human tissues. Human breast tumors showed a ∼7-fold elevation in Kindlin-3 mRNA compared with nonneoplastic tissue by quantitative polymerase chain reaction. Kindlin-3 overexpression in a breast cancer cell line increased primary tumor growth and lung metastasis by 2.5- and 3-fold, respectively, when implanted into mice compared with cells expressing vector alone. Mechanistically, the Kindlin-3-overexpressing cells displayed a 2.2-fold increase in vascular endothelial growth factor (VEGF) secretion and enhanced β1 integrin activation. Increased VEGF secretion resulted from enhanced production of Twist, a transcription factor that promotes tumor angiogenesis. Knockdown of Twist diminished VEGF production, and knockdown of β1 integrins diminished Twist and VEGF production by Kindlin-3-overexpressing cells, while nontargeting small interfering RNA had no effect on expression of these gene products. Thus, Kindlin-3 influences breast cancer progression by influencing the crosstalk between β1 integrins and Twist to increase VEGF production. This signaling cascade enhances breast cancer cell invasion and tumor angiogenesis and metastasis.
Keywords: VEGF; VEGFR2; epithelial-to-mesenchymal-transition; integrins; tumor macrophages.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

