Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans
- PMID: 24470469
- PMCID: PMC3993402
- DOI: 10.1128/IAI.01089-13
Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans
Abstract
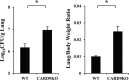
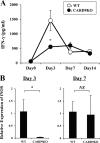
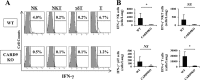
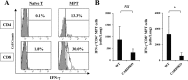
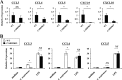
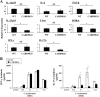
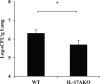
Caspase recruitment domain-containing protein 9 (CARD9) is an adaptor molecule signal that is critical for NF-κB activation and is triggered through C-type lectin receptors (CLRs), which are pattern recognition receptors that recognize carbohydrate structures. Previous studies have reported that Cryptococcus neoformans, a fungal pathogen that causes meningoencephalitis in AIDS patients, is recognized through some CLRs, such as mannose receptors or DC-SIGN. However, the role of CARD9 in the host defense against cryptococcal infection remains to be elucidated. In the present study, we analyzed the role of CARD9 in the host defense against pulmonary infection with C. neoformans. CARD9 gene-disrupted (knockout [KO]) mice were highly susceptible to this infection, as shown by the reduced fungal clearance in the infected lungs of CARD9 KO mice, compared to that in wild-type (WT) mice. Gamma interferon (IFN-γ) production was strongly reduced in CARD9 KO mice during the innate-immunity phase of infection. Reduced IFN-γ synthesis was due to impaired accumulation of NK and memory phenotype T cells, which are major sources of IFN-γ innate-immunity-phase production; a reduction in the accumulation of these cells was correlated with reduced CCL4, CCL5, CXCL9, and CXCL10 synthesis. However, differentiation of Th17 cells, but not of Th1 cells, was impaired at the adaptive-immunity phase in CARD9 KO mice compared to WT mice, although there was no significant difference in the infection susceptibility between interleukin 17A (IL-17A) KO and WT mice. These results suggest that CARD9 KO mice are susceptible to C. neoformans infection probably due to the reduced accumulation of IFN-γ-expressing NK and memory phenotype T cells at the early stage of infection.
Figures


Similar articles
-
CARD9 Is Required for Classical Macrophage Activation and the Induction of Protective Immunity against Pulmonary Cryptococcosis.mBio. 2020 Jan 7;11(1):e03005-19. doi: 10.1128/mBio.03005-19. mBio. 2020. PMID: 31911495 Free PMC article.
-
Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production.Infect Immun. 2014 Mar;82(3):937-48. doi: 10.1128/IAI.01477-13. Epub 2013 Dec 9. Infect Immun. 2014. PMID: 24324191 Free PMC article.
-
Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis.PLoS One. 2009 Sep 3;4(9):e6854. doi: 10.1371/journal.pone.0006854. PLoS One. 2009. PMID: 19727388 Free PMC article.
-
[Up-to-date findings in the host defence mechanism to cryptococcus infection].Med Mycol J. 2014;55(3):J107-14. doi: 10.3314/mmj.55.j107. Med Mycol J. 2014. PMID: 25231225 Review. Japanese.
-
Regulation by innate immune T lymphocytes in the host defense against pulmonary infection with Cryptococcus neoformans.Jpn J Infect Dis. 2004 Aug;57(4):137-45. Jpn J Infect Dis. 2004. PMID: 15329444 Review.
Cited by
-
DAP12 Inhibits Pulmonary Immune Responses to Cryptococcus neoformans.Infect Immun. 2016 May 24;84(6):1879-86. doi: 10.1128/IAI.00222-16. Print 2016 Jun. Infect Immun. 2016. PMID: 27068093 Free PMC article.
-
During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk.Cell Host Microbe. 2020 Jul 8;28(1):104-116.e4. doi: 10.1016/j.chom.2020.05.002. Epub 2020 Jun 1. Cell Host Microbe. 2020. PMID: 32485165 Free PMC article.
-
Innate Immunity against Cryptococcus, from Recognition to Elimination.J Fungi (Basel). 2018 Mar 7;4(1):33. doi: 10.3390/jof4010033. J Fungi (Basel). 2018. PMID: 29518906 Free PMC article. Review.
-
CARD9 in host immunity to fungal, bacterial, viral, and parasitic infections: An update.Front Microbiol. 2022 Nov 9;13:1021837. doi: 10.3389/fmicb.2022.1021837. eCollection 2022. Front Microbiol. 2022. PMID: 36439825 Free PMC article. Review.
-
Limited Role of Mincle in the Host Defense against Infection with Cryptococcus deneoformans.Infect Immun. 2020 Oct 19;88(11):e00400-20. doi: 10.1128/IAI.00400-20. Print 2020 Oct 19. Infect Immun. 2020. PMID: 32868343 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

