PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels
- PMID: 24470487
- PMCID: PMC4001779
- DOI: 10.1085/jgp.201311033
PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels
Abstract
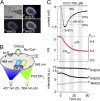
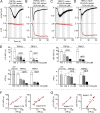
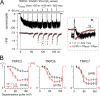
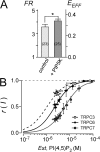
Transient receptor potential classical (or canonical) (TRPC)3, TRPC6, and TRPC7 are a subfamily of TRPC channels activated by diacylglycerol (DAG) produced through the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) by phospholipase C (PLC). PI(4,5)P2 depletion by a heterologously expressed phosphatase inhibits TRPC3, TRPC6, and TRPC7 activity independently of DAG; however, the physiological role of PI(4,5)P2 reduction on channel activity remains unclear. We used Förster resonance energy transfer (FRET) to measure PI(4,5)P2 or DAG dynamics concurrently with TRPC6 or TRPC7 currents after agonist stimulation of receptors that couple to Gq and thereby activate PLC. Measurements made at different levels of receptor activation revealed a correlation between the kinetics of PI(4,5)P2 reduction and those of receptor-operated TRPC6 and TRPC7 current activation and inactivation. In contrast, DAG production correlated with channel activation but not inactivation; moreover, the time course of channel inactivation was unchanged in protein kinase C-insensitive mutants. These results suggest that inactivation of receptor-operated TRPC currents is primarily mediated by the dissociation of PI(4,5)P2. We determined the functional dissociation constant of PI(4,5)P2 to TRPC channels using FRET of the PLCδ Pleckstrin homology domain (PHd), which binds PI(4,5)P2, and used this constant to fit our experimental data to a model in which channel gating is controlled by PI(4,5)P2 and DAG. This model predicted similar FRET dynamics of the PHd to measured FRET in either human embryonic kidney cells or smooth muscle cells, whereas a model lacking PI(4,5)P2 regulation failed to reproduce the experimental data, confirming the inhibitory role of PI(4,5)P2 depletion on TRPC currents. Our model also explains various PLC-dependent characteristics of channel activity, including limitation of maximum open probability, shortening of the peak time, and the bell-shaped response of total current. In conclusion, our studies demonstrate a fundamental role for PI(4,5)P2 in regulating TRPC6 and TRPC7 activity triggered by PLC-coupled receptor stimulation.
Figures



Comment in
-
Bridge between the channel and FRET of PtdIns(4,5)P₂ sensor.Channels (Austin). 2014;8(4):292-3. doi: 10.4161/chan.29967. Channels (Austin). 2014. PMID: 25478620 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

