Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02
- PMID: 24470557
- PMCID: PMC3956354
- DOI: 10.1093/neuonc/not247
Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02
Abstract
Background: Inhibition of epidermal growth factor receptor (EGFR) and the mechanistic target of rapamycin (mTOR) may have synergistic antitumor effects in high-grade glioma patients.
Methods: We conducted a phase I/II study of the EGFR inhibitor erlotinib (150 mg/day) and the mTOR inhibitor temsirolimus. Patients initially received temsirolimus 50 mg weekly, and the dose adjusted based on toxicities. In the phase II component, the primary endpoint was 6-month progression-free survival (PFS6) among glioblastoma patients.
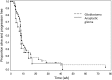
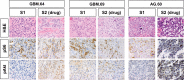
Results: Twenty-two patients enrolled in phase I, 47 in phase II. Twelve phase I patients treated at the maximum tolerated dosage were included in the phase II cohort for analysis. The maximum tolerated dosage was 15 mg temsirolimus weekly with erlotinib 150 mg daily. Dose-limiting toxicities were rash and mucositis. Among 42 evaluable glioblastoma patients, 12 (29%) achieved stable disease, but there were no responses, and PFS6 was 13%. Among 16 anaplastic glioma patients, 1 (6%) achieved complete response, 1 (6%) partial response, and 2 (12.5%) stable disease, with PFS6 of 8%. Tumor levels of both drugs were low, and posttreatment tissue in 3 patients showed no reduction in the mTOR target phosphorylated (phospho-)S6(S235/236) but possible compensatory increase in phospho-Akt(S473). Presence of EGFR variant III, phospho-EGFR, and EGFR amplification did not correlate with survival, but patients with elevated phospho-extracellular signal-regulated kinase or reduced phosphatase and tensin homolog protein expression had decreased progression-free survival at 4 months.
Conclusion: Because of increased toxicity, the maximum tolerated dosage of temsirolimus in combination with erlotinib proved lower than expected. Insufficient tumor drug levels and redundant signaling pathways may partly explain the minimal antitumor activity noted.
Keywords: anaplastic glioma; clinical trial; epidermal growth factor; erlotinib; glioblastoma; temsirolimus.
Figures
Similar articles
-
A dose escalation trial for the combination of erlotinib and sirolimus for recurrent malignant gliomas.J Neurooncol. 2012 Nov;110(2):245-50. doi: 10.1007/s11060-012-0960-y. Epub 2012 Aug 24. J Neurooncol. 2012. PMID: 22918789 Free PMC article. Clinical Trial.
-
Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma.Neuro Oncol. 2010 Dec;12(12):1300-10. doi: 10.1093/neuonc/noq099. Epub 2010 Aug 17. Neuro Oncol. 2010. PMID: 20716591 Free PMC article. Clinical Trial.
-
Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme.Anticancer Res. 2013 Apr;33(4):1657-60. Anticancer Res. 2013. PMID: 23564811 Clinical Trial.
-
Phase 1/2 trial of temsirolimus and sorafenib in the treatment of patients with recurrent glioblastoma: North Central Cancer Treatment Group Study/Alliance N0572.Cancer. 2018 Apr 1;124(7):1455-1463. doi: 10.1002/cncr.31219. Epub 2018 Jan 3. Cancer. 2018. PMID: 29313954 Free PMC article. Clinical Trial.
-
Glioma: challenges and new insights in the development of effective therapies.Hawaii Med J. 2008 Jan;67(1):20-3, 25. Hawaii Med J. 2008. PMID: 18309836 Review. No abstract available.
Cited by
-
Genomic and Functional Analysis of the E3 Ligase PARK2 in Glioma.Cancer Res. 2015 May 1;75(9):1815-27. doi: 10.1158/0008-5472.CAN-14-1433. Epub 2015 Apr 15. Cancer Res. 2015. PMID: 25877876 Free PMC article.
-
Finding the Right Way to Target EGFR in Glioblastomas; Lessons from Lung Adenocarcinomas.Cancers (Basel). 2018 Dec 4;10(12):489. doi: 10.3390/cancers10120489. Cancers (Basel). 2018. PMID: 30518123 Free PMC article.
-
Glioblastoma pharmacotherapy: A multifaceted perspective of conventional and emerging treatments (Review).Exp Ther Med. 2021 Dec;22(6):1408. doi: 10.3892/etm.2021.10844. Epub 2021 Oct 6. Exp Ther Med. 2021. PMID: 34676001 Free PMC article. Review.
-
Molecular pathologic diagnosis of epidermal growth factor receptor.Neuro Oncol. 2014 Oct;16 Suppl 8(Suppl 8):viii1-6. doi: 10.1093/neuonc/nou294. Neuro Oncol. 2014. PMID: 25342599 Free PMC article. Review.
-
EGFR as a Target for Glioblastoma Treatment: An Unfulfilled Promise.CNS Drugs. 2017 Sep;31(9):723-735. doi: 10.1007/s40263-017-0456-6. CNS Drugs. 2017. PMID: 28791656 Free PMC article. Review.
References
-
- CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. Hinsdale IL: Central Brain Tumor Registry of the United States; 2011.
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. - PubMed
-
- Nicholas MK, Lukas RV, Jafri NF, et al. Epidermal growth factor receptor–mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res. 2006;12:7261–7270. - PubMed
-
- Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

