Collagenase clostridium histolyticum in patients with Dupuytren's contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes
- PMID: 24470559
- PMCID: PMC4361452
- DOI: 10.1177/1753193413519926
Collagenase clostridium histolyticum in patients with Dupuytren's contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes
Abstract
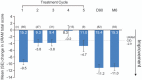
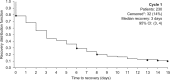
In POINT X, a study designed to reflect clinical practice and patient treatment choices, 254 European patients received open-label collagenase for Dupuytren's contracture. The most severely affected joint was treated first in 74% of patients. In total, 52%, 41%, 7%, and 1% of patients selected the little, ring, middle, and index finger, respectively; 79% had one or two joints treated. Only 9% of patients (n = 24) received 4 or 5 injections. The mean improvement in total passive extension deficit (TPED) was 34° on day 1, improving further by day 7 to 42°. This secondary improvement was maintained by day 90 and month 6. The mean number of injections/joint was 1.2 for the metacarpophalangeal joint and 1.25 for the proximal interphalangeal joint. Median time to recovery was 4 days; the mean improvement in hand function was clinically relevant as measured by the Unité Rhumatologique des Affections de la Main (URAM) score. In total, 87% and 86% of patients and physicians, respectively, were very satisfied or satisfied with treatment at month 6, although correlation between TPED and patient satisfaction was weak (Spearman -0.18, 95% CI -0.32 to -0.06). Collagenase was well tolerated, with 10 (3.9%) patients experiencing severe adverse events. As a real-world study, the POINT X findings can be generalized to the at-large population.
Trial registration: ClinicalTrials.gov NCT01229436.
Keywords: Collagenase; Dupuytren’s disease; efficacy; open label; patient-reported outcomes; tolerability.
© The Author(s) 2014.
Conflict of interest statement
Figures


References
-
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007, 32: 767–74. - PubMed
-
- Beaudreuil J, Allard A, Zerkak D, et al. ; URAM Study Group. Unité Rhumatologique des Affections de la Main (URAM) scale: development and validation of a tool to assess Dupuytren’s disease-specific disability. Arthritis Care Res (Hoboken). 2011, 63: 1448–55. - PubMed
-
- Council for International Organizations of Medical Sciences, World Health Organization. International ethical guidelines for biomedical research involving human subjects. http://www.cioms.ch/images/stories/CIOMS/guidelines/guidelines_nov_2002_... (26 November 2013). - PubMed
-
- Gilpin D, Coleman S, Hall S, et al. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s contracture. J Hand Surg Am. 2010, 35: 2027–38.e1. - PubMed
-
- Hay DC, Louie DL, Earp BE, Kaplan FTD, Akelman E, Blazar PE. Surgical findings in the treatment of Dupuytren’s disease after initial treatment with clostridial collagenase (Xiaflex). J Hand Surg Eur. Epub ahead of print 6 May, 2013. DOI 1753193413488305. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

