Efficacy of a reduced pill burden on therapeutic adherence to calcineurin inhibitors in renal transplant recipients: an observational study
- PMID: 24470756
- PMCID: PMC3891638
- DOI: 10.2147/PPA.S54922
Efficacy of a reduced pill burden on therapeutic adherence to calcineurin inhibitors in renal transplant recipients: an observational study
Abstract
Purpose: The aim of this study was to determine the prevalence of nonadherence in a cohort of renal transplant recipients (RTRs) and to evaluate prospectively whether more intense clinical surveillance and reduced pill number enhanced adherence.
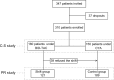
Patients and methods: The study was carried out in 310 stable RTRs in whom adherence, life satisfaction, and transplant care were evaluated by specific questionnaires (time 0). The patients under tacrolimus (TAC; bis in die [BID]) were then shifted to once-daily TAC (D-TAC) to reduce their pill burden (Shift group) and were followed up for 6 months to reevaluate the same parameters. Patients on cyclosporin or still on BID-TAC constituted a time-control group.
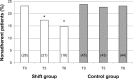
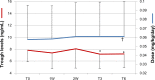
Results: The prevalence of nonadherence was 23.5% and was associated with previous rejection episodes (P<0.002), and was inversely related to Life Satisfaction Index, anxiety, and low glomerular filtration rate (minimum P<0.03). Nonadherent patients were significantly less satisfied with their medical care and their relationships with the medical staff. A shift from BID-TAC to D-TAC was performed in 121 patients, and the questionnaires were repeated after 3 and 6 months. In the Shift group, a reduction in pill number was observed (P<0.01), associated with improved adherence after 3 and 6 months (+36%, P<0.05 versus basal), with no change in controls. Decreased TAC trough levels after 3 and 6 months (-9%), despite a slight increase in drug dosage (+6.5%), were observed in the Shift group, with no clinical side effects.
Conclusion: The reduced pill burden improves patients' compliance to calcineurin-inhibitors, but major efforts in preventing nonadherence are needed.
Keywords: adherence; calcineurin inhibitors; once-daily tacrolimus; renal transplant.
Figures
References
-
- World Health Organization . Adherence to Long-Term Therapies: Evidence for Action. Geneva: WHO; 2003. [Accessed September 12, 2012]. Available from: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf.
-
- Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858–873. - PubMed
-
- Siegal BR, Greenstein SM. Postrenal transplant compliance from the perspective of African-Americans, Hispanic-Americans, and Anglo-Americans. Adv Ren Replace Ther. 1997;4:46–54. - PubMed
-
- Greenstein S, Siegal B. Compliance and noncompliance in patients with a functional renal transplant: a multicenter study. Transplantation. 1998;66:1718–1726. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

