Nanoparticles containing siRNA to silence CD4 and CCR5 reduce expression of these receptors and inhibit HIV-1 infection in human female reproductive tract tissue explants
- PMID: 24470908
- PMCID: PMC3892589
- DOI: 10.4081/idr.2011.e11
Nanoparticles containing siRNA to silence CD4 and CCR5 reduce expression of these receptors and inhibit HIV-1 infection in human female reproductive tract tissue explants
Abstract
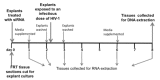
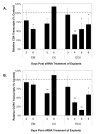
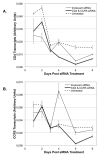
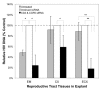
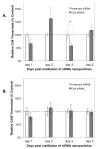
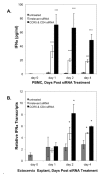
Human Immunodeficiency Virus-type 1 (HIV-1) binds to CD4 and CCR5 receptors on target cells in the human female reproductive tract. We sought to determine whether reducing levels of messenger RNA (mRNA) transcripts that encode these receptors in female reproductive tract cells could protect mucosal tissue explants from HIV-1 infection. Explants prepared from the endometrium, endocervix, and ectocervix of hysterectomy tissues from HIV-1 sero-negative women were exposed to nanoparticles containing CD4- and CCR5-specific short-interfering RNA (siRNA) sequences. Explants were then exposed two days later to HIV-1, and HIV-1 reverse transcripts were measured five days post-infection. Explants treated with nanoparticles containing CD4- and CCR5-specific siRNA showed reduced levels of CD4 and CCR5 transcripts, and significantly lower levels of HIV-1 reverse transcripts compared to those treated with an irrelevant siRNA. In female reproductive tract explants and in peripheral blood cell cultures, siRNA transfection induced the secretion of IFN-alpha (IFN-α), a potent antiviral cytokine. In female mice, murine-specific Cd4-siRNA nanoparticles instilled within the uterus significantly reduced murine Cd4 transcripts by day 3. Our findings demonstrate that siRNA nanoparticles reduce expression of HIV-1 infectivity receptors in human female reproductive tract tissues and also inhibit HIV-1 infection. Murine studies demonstrate that nanoparticles can penetrate the reproductive tract tissues in vivo and silence gene expression. The induction of IFN-α after siRNA transfection can potentially contribute to the antiviral effect. These findings support the therapeutic development of nanoparticles to deliver siRNA molecules to silence host cell receptors in the female reproductive tract as a novel microbicide to inhibit mucosal HIV-1 transmission.
Keywords: HIV-1; heterosexual transmission; nanoparticles.; virus infection; women.
Conflict of interest statement
Conflict of interest: the authors report no conflicts of interest.
Figures
References
-
- Nunnari G, Otero M, Dornadula G, et al. Residual HIV-1 disease in seminal cells of HIV-1-infected men on suppressive HAART: latency without on-going cellular infections. AIDS. 2002;16:39–45. - PubMed
-
- Kiessling A. Isolation of human immunod-eficiency virus type 1 from semen and vaginal fluids. Methods Mol Biol. 2005;304:71–86. - PubMed
-
- Ball J, Curran R, Irving W, Dearden A. HIV-1 in semen: determination of proviral and viral titres compared to blood, and quantification of semen leukocyte populations. J Med Virol. 1999;59:356–63. - PubMed
-
- Suresh P, Wanchu A. Chemokines and chemokine receptors in HIV infection: role in pathogenesis and therapeutics. J Postgrad Med. 2006;52:210–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

