Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways
- PMID: 24471696
- PMCID: PMC4091855
- DOI: 10.1165/rcmb.2013-0284OC
Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways
Abstract
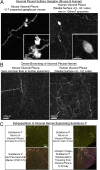
In whole adult mouse lung, full identification of airway nerves (or other cellular/subcellular objects) has not been possible due to patchy distribution and micron-scale size. Here we describe a method using tissue clearing to acquire the first complete image of three-dimensional (3D) innervation in the lung. We then created a method to pair analysis of nerve (or any other colabeled epitope) images with identification of 3D tissue compartments and airway morphometry by using fluorescent casting and morphometry software (which we designed and are making available as open-source). We then tested our method to quantify a sparse heterogeneous nerve population by examining visceral pleural nerves. Finally, we demonstrate the utility of our method in human tissue to image full thickness innervation in irregular 3D tissue compartments and to quantify sparse objects (intrinsic airway ganglia). Overall, this method can uniquely pair the advantages of whole tissue imaging and cellular/subcellular fluorescence microscopy.
Keywords: clearing; modeling; morphometry; nerve; visceral pleura.
Figures






References
-
- Muhlfeld C, Graulich T, Das SK, Krasteva G, Wessels L, Ruppert C, Kummer W, Hofler G.Tracheal innervation and alveolar epithelial type II cell lamellar bodies are reduced in the cancer cachectic mouse Am J Respir Crit Care Med 2012. [accessed 2013 Oct 1]. Available from: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2012.185.1_... - DOI
-
- O’Connell F, Springall DR, Moradoghli-Haftvani A, Krausz T, Price D, Fuller RW, Polak JM, Pride NB. Abnormal intraepithelial airway nerves in persistent unexplained cough? Am J Respir Crit Care Med. 1995;152:2068–2075. - PubMed
-
- Ollerenshaw SL, Jarvis D, Sullivan CE, Woolcock AJ. Substance P immunoreactive nerves in airways from asthmatics and nonasthmatics. Eur Respir J. 1991;4:673–682. - PubMed
-
- Howarth PH, Springall DR, Redington AE, Djukanovic R, Holgate ST, Polak JM. Neuropeptide-containing nerves in endobronchial biopsies from asthmatic and nonasthmatic subjects. Am J Respir Cell Mol Biol. 1995;13:288–296. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

