Night-time sedating H1 -antihistamine increases daytime somnolence but not treatment efficacy in chronic spontaneous urticaria: a randomized controlled trial
- PMID: 24472058
- PMCID: PMC4232862
- DOI: 10.1111/bjd.12846
Night-time sedating H1 -antihistamine increases daytime somnolence but not treatment efficacy in chronic spontaneous urticaria: a randomized controlled trial
Abstract
Background: Many physicians believe that the most effective way to treat chronic urticaria is to take a nonsedating second-generation H1 -antihistamine in the morning and a sedating first-generation H1 -antihistamine, usually hydroxyzine, at night to enhance sleep. But is this belief well founded?
Objectives: To test this belief by comparing the effectiveness and prevalence of unwanted sedative effects when treating patients with chronic spontaneous urticaria (CSU) with levocetirizine 15 mg daily plus hydroxyzine 50 mg at night (levocetirizine plus hydroxyzine) vs. levocetirizine 20 mg daily (levocetirizine monotherapy).
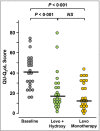
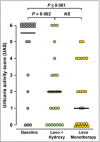
Methods: In this randomized, double-blind, cross-over study, 24 patients with difficult-to-treat CSU took levocetirizine plus hydroxyzine or levocetirizine monotherapy for periods of 5 days each. At the end of each treatment period, assessments were made of quality of life (Chronic Urticaria Quality of Life Questionnaire, CU-Q2 oL), severity of urticaria symptoms (Urticaria Activity Score, UAS), sleep disturbance during the night and daytime somnolence.
Results: Both treatments significantly decreased UAS, night-time sleep disturbances and CU-Q2 oL scores (P < 0·001) without significant differences between the two. Compared with baseline, daytime somnolence was significantly reduced by levocetirizine monotherapy (P = 0·006) but not by levocetirizine plus hydroxyzine (P = 0·218). Direct comparison of the two treatment modalities in terms of daytime somnolence favoured levocetirizine monotherapy (P = 0·026).
Conclusions: The widespread belief that sleep is aided by the addition of a sedating first-generation H1 -antihistamine, usually hydroxyzine, at night is not supported. These results are in line with the urticaria guidelines, which state that first-line treatment for urticaria should be new-generation, nonsedating H1 -antihistamines only.
© 2014 The Authors. British Association of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures


Comment in
-
Night-time sedating H1 antihistamine increases daytime somnolence but not treatment efficacy in chronic spontaneous urticaria: a randomized controlled study.Br J Dermatol. 2014 Jul;171(1):8-9. doi: 10.1111/bjd.13112. Br J Dermatol. 2014. PMID: 25066287 No abstract available.
References
-
- Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66:317–30. - PubMed
-
- O’Donnell BF, Lawlor F, Simpson J, et al. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136:197–201. - PubMed
-
- Baiardini I, Pasquali M, Braido F, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL) Allergy. 2005;60:1073–8. - PubMed
-
- Murota H, Kitaba S, Tani M, et al. Effects of nonsedative antihistamines on productivity of patients with pruritic skin diseases. Allergy. 2010;65:929–30. - PubMed
-
- Zuberbier T, Asero R, Bindslev-Jensen C, et al. EAACI/GA2LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

