Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons
- PMID: 24472174
- PMCID: PMC3909300
- DOI: 10.1186/1744-8069-10-8
Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons
Abstract
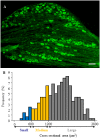
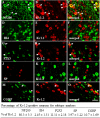
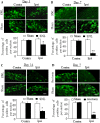
Voltage-gated potassium (Kv) channels are critical in controlling neuronal excitability and are involved in the induction of neuropathic pain. Therefore, Kv channels might be potential targets for prevention and/or treatment of this disorder. We reported here that a majority of dorsal root ganglion (DRG) neurons were positive for Kv channel alpha subunit Kv1.2. Most of them were large and medium, although there was a variety of sizes. Peripheral nerve injury caused by lumbar (L)5 spinal nerve ligation (SNL) produced a time-dependent reduction in the number of Kv1.2-positive neurons in the ipsilateral L5 DRG, but not in the contralateral L5 DRG. Such reduction was also observed in the ipsilateral L5 DRG on day 7 after sciatic nerve axotomy. Rescuing nerve injury-induced reduction of Kv1.2 in the injured L5 DRG attenuated the development and maintenance of SNL-induced pain hypersensitivity without affecting acute pain and locomotor function. This effect might be attributed to the prevention of SNL-induced upregulation of endogenous Kv1.2 antisense RNA, in addition to the increase in Kv1.2 protein expression, in the injured DRG. Our findings suggest that Kv1.2 may be a novel potential target for preventing and/or treating neuropathic pain.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

