Hypoxia enhances protective effect of placental-derived mesenchymal stem cells on damaged intestinal epithelial cells by promoting secretion of insulin-like growth factor-1
- PMID: 24473145
- PMCID: PMC3958833
- DOI: 10.3390/ijms15021983
Hypoxia enhances protective effect of placental-derived mesenchymal stem cells on damaged intestinal epithelial cells by promoting secretion of insulin-like growth factor-1
Abstract
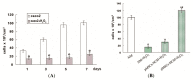
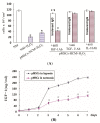
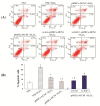
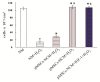
Apoptosis and necrosis of intestinal epithelial cells (IECs), induced by ischemia-reperfusion (I/R) injury, can lead to dysfunction of the intestinal barrier, which could cause multiple organ dysfunction syndromes. Mesenchymal stem cells (MSCs) have the potential of providing protective effects on damaged IECs via paracrine action. This study investigated whether hypoxia can enhance the protective effect of placental-derived MSCs (pMSCs) on H2O2-treated-caco2 cells, and explored the possible mechanism. The pMSCs isolated by tissue culture were fibroblast-like, positive for CD73, CD90 and CD105 and can differentiate into chondrocytes and endothelial cells. Five days after treatment with H2O2, the numbers of living caco2 cells significantly decreased. More live H2O2-treated-caco2 cells were observed in pMSCs hypoxia culture medium (pMSCs-HCM) than pMSCs normoxia culture medium (pMSCs-NCM), and the application of a specific antibody that blocked insulin-like growth factor-1 (IGF-1) leads to a significant decrease of the protective effect of pMSCs-HCM. Hypoxia can promote IGF-1 expression of pMSCs at mRNA and protein levels, and caco2 stably expressed IGF-1 receptor. Knocking down IGF-1 expression in pMSCs by siRNA resulted in a significant attenuation of the increase in apoptosis of H2O2-treated-caco2 cultured in pMSCs-HCM. In conclusion, hypoxia can increase the protective effect of pMSCs on H2O2-treated-caco2 cells via a promotion of their paracrine actions, and the key cytokine involved is IGF-1.
Figures




Similar articles
-
Human chorionic villous mesenchymal stem/stromal cells protect endothelial cells from injury induced by high level of glucose.Stem Cell Res Ther. 2018 Sep 21;9(1):238. doi: 10.1186/s13287-018-0984-0. Stem Cell Res Ther. 2018. PMID: 30241570 Free PMC article.
-
Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation.Life Sci. 2016 Mar 15;149:51-7. doi: 10.1016/j.lfs.2016.02.050. Epub 2016 Feb 15. Life Sci. 2016. PMID: 26892145
-
Mesenchymal Stem/Stromal Cells from the Placentae of Growth Restricted Pregnancies Are Poor Stimulators of Angiogenesis.Stem Cell Rev Rep. 2020 Jun;16(3):557-568. doi: 10.1007/s12015-020-09959-8. Stem Cell Rev Rep. 2020. PMID: 32080795
-
Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation.Stem Cell Rev Rep. 2009 Sep;5(3):247-55. doi: 10.1007/s12015-009-9069-x. Epub 2009 May 23. Stem Cell Rev Rep. 2009. PMID: 19590988 Review.
-
Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis.Cell Mol Life Sci. 2020 Jan;77(2):253-265. doi: 10.1007/s00018-019-03268-1. Epub 2019 Aug 29. Cell Mol Life Sci. 2020. PMID: 31468060 Free PMC article. Review.
Cited by
-
The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke.J Cereb Blood Flow Metab. 2018 Aug;38(8):1276-1292. doi: 10.1177/0271678X18776802. Epub 2018 May 17. J Cereb Blood Flow Metab. 2018. PMID: 29768965 Free PMC article. Review.
-
Targeting cell death pathways in intestinal ischemia-reperfusion injury: a comprehensive review.Cell Death Discov. 2024 Mar 4;10(1):112. doi: 10.1038/s41420-024-01891-x. Cell Death Discov. 2024. PMID: 38438362 Free PMC article. Review.
-
Insulin-like growth factor 1 receptor-mediated cell survival in hypoxia depends on the promotion of autophagy via suppression of the PI3K/Akt/mTOR signaling pathway.Mol Med Rep. 2017 Apr;15(4):2136-2142. doi: 10.3892/mmr.2017.6265. Epub 2017 Mar 1. Mol Med Rep. 2017. PMID: 28260056 Free PMC article.
-
Bone marrow mesenchymal stem cell-derived exosomes promote osteoblast proliferation, migration and inhibit apoptosis by regulating KLF3-AS1/miR-338-3p.BMC Musculoskelet Disord. 2024 Feb 9;25(1):122. doi: 10.1186/s12891-024-07236-0. BMC Musculoskelet Disord. 2024. PMID: 38336637 Free PMC article.
-
Conditioned medium from primary cytotrophoblasts, primary placenta-derived mesenchymal stem cells, or sub-cultured placental tissue promoted HUVEC angiogenesis in vitro.Stem Cell Res Ther. 2021 Feb 17;12(1):141. doi: 10.1186/s13287-021-02192-1. Stem Cell Res Ther. 2021. PMID: 33596987 Free PMC article.
References
-
- Oldeburg W.A., Lau L.L., Rodenberg T.J., Edmonds H.J., Burger C.D. Acute mesenteric ischemia: A clinical review. Arch. Intern. Med. 2004;164:1054–1062. - PubMed
-
- Quaedackers J.S., Beukm R.J., Bennetm L., Charlton A., Oude Eqbrink M.G., Gunn A.J., Heineman E. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant. Proc. 2000;32:1307–1310. - PubMed
-
- Khanna A., Rossman J., Caty M.G., Fung H.L. Beneficial effects of intraluminal nitroglycerin in intestinal ischemia-reperfusion injury in rats. J. Surg. Res. 2003;114:5–24. - PubMed
-
- Wang H.J., Huang J. Basic and applicative studies of acidic fibroblast growth factor. Anat. Res. 2005;2:308–311.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

