Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects
- PMID: 24473225
- PMCID: PMC3926796
- DOI: 10.1634/theoncologist.2013-0359
Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects
Abstract
Objective: Our objective was to evaluate preferences associated with grade I/II and grade III/IV chemotherapy side effects among breast cancer patients receiving chemotherapy. We also assessed trade-offs that patients are willing to make between treatment side effects and the route and schedule of treatment administration.
Methods: In this cross-sectional study, patients receiving chemotherapy for breast cancer completed a one-time Web survey. Conjoint analysis was used to elicit preferences for 17 grade I/II and III/IV side effects associated with available chemotherapies and regimens. In the analysis, the risk of each side effect was increased by 5%, holding all others constant, and the respective impact on patient preferences was identified.
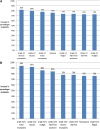
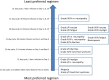
Results: A total of 102 women participated (mean age 54 ± 11). Among the grade I/II side effects, a 5% reduction in the risk of sensory neuropathy, nausea, and motor neuropathy had the highest impact on preferences. Among grade III/IV side effects, motor neuropathy, nausea/vomiting, and myalgia made the most difference. An oral twice-daily regimen was most preferred; however, patients were willing to receive an intravenous regimen relative to oral to avoid an increased risk of 5% in the majority of side effects. Avoiding an increased chance of grade III/IV motor neuropathy was associated with willingness to tolerate one of the least preferred administration schedules.
Conclusion: This study identified relative preferences among both mild/moderate to severe side effects from the patient perspective. Patients appear to be willing to make trade-offs between side effects and different regimens. These findings may help to inform medical decision-making processes.
Keywords: Breast cancer; Conjoint; Preferences; Side effects; Tradeoffs.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. The Oncologist. 2003;8:514–520. - PubMed
-
- Young A, Topham C, Moore J, et al. A patient preference study comparing raltitrexed (‘Tomudex’) and bolus or infusional 5-fluorouracil regimens in advanced colorectal cancer: Influence of side-effects and administration attributes. Eur J Cancer Care. 1999;8:154–161. - PubMed
-
- Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13:219–227. - PubMed
-
- Dubey S, Brown RL, Esmond SL, et al. Patient preferences in choosing chemotherapy regimens for advanced non-small cell lung cancer. J Support Oncol. 2005;3:149–154. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

