Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study
- PMID: 24473673
- PMCID: PMC3995223
- DOI: 10.1136/annrheumdis-2013-204761
Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study
Abstract
Objective: To assess the 1-year efficacy and safety of a regimen of tocilizumab plus methotrexate or placebo, which was augmented by a treat-to-target strategy from week 24.
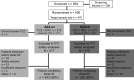
Methods: ACT-RAY was a double-blind, 3-year trial. Adults with active rheumatoid arthritis despite methotrexate were randomised to add tocilizumab to ongoing methotrexate (add-on strategy) or to switch to tocilizumab plus placebo (switch strategy). Tocilizumab 8 mg/kg was administered every 4 weeks. Conventional open-label disease-modifying antirheumatic drugs (DMARDs) other than methotrexate were added at week 24 or later in patients with DAS28>3.2.
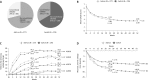
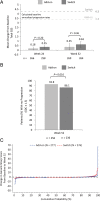
Results: 556 patients were randomised; 85% completed 52 weeks. The proportion of patients receiving open-label DMARDs was comparable in the add-on (29%) and switch (33%) arms. Overall, week 24 results were maintained or further improved at week 52 in both arms. Some endpoints favoured the add-on strategy. Mean changes in Genant-modified Sharp scores were small; more add-on (92.8%) than switch patients (86.1%) had no radiographic progression. At week 52, comparable numbers of patients had antidrug antibodies (ADAs; 1.5% and 2.2% of add-on and switch patients, respectively) and neutralising ADAs (0.7% and 1.8%). Rates of serious adverse events and serious infections per 100 patient-year (PY) were 11.3 and 4.5 in add-on and 16.8 and 5.5 in switch patients. In patients with normal baseline values, alanine aminotransferase elevations >3× upper limit of normal were observed in 11% of add-on and 3% of switch patients.
Conclusions: Despite a trend favouring the add-on strategy, these data suggest that both tocilizumab add-on and switch strategies led to meaningful clinical and radiographic responses.
Keywords: DMARDs (biologic); Methotrexate; Rheumatoid Arthritis.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

