Structure-based design of novel human Toll-like receptor 8 agonists
- PMID: 24474703
- PMCID: PMC4105021
- DOI: 10.1002/cmdc.201300573
Structure-based design of novel human Toll-like receptor 8 agonists
Abstract
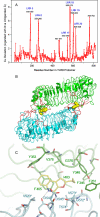
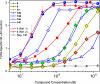
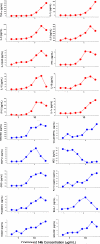
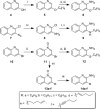
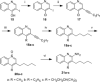
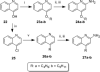
Toll-like receptor (TLR)-8 agonists activate adaptive immune responses by inducing robust production of T helper 1-polarizing cytokines, suggesting that TLR8-active compounds might be promising candidate vaccine adjuvants. Recently, a C2-butyl furo[2,3-c]quinoline was reported with purely TLR8 agonistic activity. This compound was successfully co-crystallized with the human TLR8 ectodomain, and the co-crystal structure revealed ligand-induced reorganization of the binding pocket of TLR8. The loss of a key hydrogen bond between the oxygen atom of the furanyl ring of the agonist and Thr 574 in TLR8 suggested that the furan ring is dispensable. Employing a disconnection strategy, 3- and 4-substituted aminoquinolines were investigated. Focused structure-based ligand design studies led to the identification of 3-pentyl-quinoline-2-amine as a novel, structurally simple, and highly potent human TLR8-specific agonist (EC50 =0.2 μM). Preliminary evaluation of this compound in ex vivo human blood assay systems revealed that it retains prominent cytokine-inducing activity. Together, these results indicate the suitability of this compound as a novel vaccine adjuvant, warranting further investigation.
Keywords: aminoquinolines; innate immunity; structure-based drug design; toll-like receptor-8 (TLR8); vaccine adjuvants.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
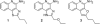
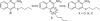
Similar articles
-
Human Toll-like receptor 8-selective agonistic activities in 1-alkyl-1H-benzimidazol-2-amines.J Med Chem. 2014 Sep 11;57(17):7325-41. doi: 10.1021/jm500701q. Epub 2014 Aug 20. J Med Chem. 2014. PMID: 25102141 Free PMC article.
-
Toll-like receptor-8 agonistic activities in C2, C4, and C8 modified thiazolo[4,5-c]quinolines.Org Biomol Chem. 2013 Feb 21;11(7):1179-98. doi: 10.1039/c2ob26705e. Org Biomol Chem. 2013. PMID: 23314908 Free PMC article.
-
Exquisite selectivity for human toll-like receptor 8 in substituted furo[2,3-c]quinolines.J Med Chem. 2013 Sep 12;56(17):6871-85. doi: 10.1021/jm400694d. Epub 2013 Aug 15. J Med Chem. 2013. PMID: 23899291 Free PMC article.
-
The use of Toll-like receptor 7/8 agonists as vaccine adjuvants.Expert Rev Vaccines. 2013 Jul;12(7):809-19. doi: 10.1586/14760584.2013.811208. Expert Rev Vaccines. 2013. PMID: 23885825 Review.
-
[Structural Analyses of Toll-like Receptor Sensing Single-stranded Nucleic Acids and Its Application].Yakugaku Zasshi. 2016;136(2):173-8. doi: 10.1248/yakushi.15-00229-1. Yakugaku Zasshi. 2016. PMID: 26831789 Review. Japanese.
Cited by
-
GlycoMinestruct: a new bioinformatics tool for highly accurate mapping of the human N-linked and O-linked glycoproteomes by incorporating structural features.Sci Rep. 2016 Oct 6;6:34595. doi: 10.1038/srep34595. Sci Rep. 2016. PMID: 27708373 Free PMC article.
-
Structural and functional understanding of the toll-like receptors.Protein Sci. 2021 Apr;30(4):761-772. doi: 10.1002/pro.4043. Epub 2021 Feb 24. Protein Sci. 2021. PMID: 33576548 Free PMC article. Review.
-
Structural evolution of toll-like receptor 7/8 agonists from imidazoquinolines to imidazoles.RSC Med Chem. 2021 May 14;12(7):1065-1120. doi: 10.1039/d1md00031d. eCollection 2021 Jul 21. RSC Med Chem. 2021. PMID: 34355178 Free PMC article. Review.
-
Human Toll-like receptor 8-selective agonistic activities in 1-alkyl-1H-benzimidazol-2-amines.J Med Chem. 2014 Sep 11;57(17):7325-41. doi: 10.1021/jm500701q. Epub 2014 Aug 20. J Med Chem. 2014. PMID: 25102141 Free PMC article.
-
Comparative Geometrical Analysis of Leucine-Rich Repeat Structures in the Nod-Like and Toll-Like Receptors in Vertebrate Innate Immunity.Biomolecules. 2015 Aug 18;5(3):1955-78. doi: 10.3390/biom5031955. Biomolecules. 2015. PMID: 26295267 Free PMC article. Review.
References
-
- Janeway CA., Jr. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. Part 1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

