Origin of regenerating tubular cells after acute kidney injury
- PMID: 24474779
- PMCID: PMC3910599
- DOI: 10.1073/pnas.1316177111
Origin of regenerating tubular cells after acute kidney injury
Abstract
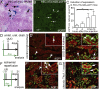
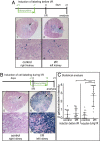
Acute kidney injury (AKI) is associated with high morbidity and mortality. Recent genetic fate mapping studies demonstrated that recovery from AKI occurs from intrinsic tubular cells. It is unresolved whether these intrinsic cells (so-called "scattered tubular cells") represent fixed progenitor cells or whether recovery involves any surviving tubular cell. Here, we show that the doxycycline-inducible parietal epithelial cell (PEC)-specific PEC-reverse-tetracycline transactivator (rtTA) transgenic mouse also efficiently labels the scattered tubular cell population. Proximal tubular cells labeled by the PEC-rtTA mouse coexpressed markers for scattered tubular cells (kidney injury molecule 1, annexin A3, src-suppressed C-kinase substrate, and CD44) and showed a higher proliferative index. The PEC-rtTA mouse labeled more tubular cells upon different tubular injuries but was independent of cellular proliferation as determined in physiological growth of the kidney. To resolve whether scattered tubular cells are fixed progenitors, cells were irreversibly labeled before ischemia reperfusion injury (genetic cell fate mapping). During recovery, the frequency of labeled tubular cells remained constant, arguing against a fixed progenitor population. In contrast, when genetic labeling was induced during ischemic injury and subsequent recovery, the number of labeled cells increased significantly, indicating that scattered tubular cells arise from any surviving tubular cell. In summary, scattered tubular cells do not represent a fixed progenitor population but rather a phenotype that can be adopted by almost any proximal tubular cell upon injury. Understanding and modulating these phenotypic changes using the PEC-rtTA mouse may lead to more specific therapies in AKI.
Keywords: cell fate tracking; lineage tracing; regeneration; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: Results from genetic labeling techniques. Kidney Int. 2011;79(5):494–501. - PubMed
-
- Little MH, Bertram JF. Is there such a thing as a renal stem cell? J Am Soc Nephrol. 2009;20(10):2112–2117. - PubMed
-
- Glaumann B, Glaumann H, Trump BF. Studies of cellular recovery from injury. III. Ultrastructural studies on the recovery of the pars recta of the proximal tubule (P3 segment) of the rat kidney from temporary ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1977;25(4):281–308. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

