Organization of the human fetal subpallium
- PMID: 24474906
- PMCID: PMC3893616
- DOI: 10.3389/fnana.2013.00054
Organization of the human fetal subpallium
Abstract
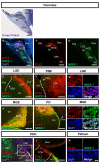
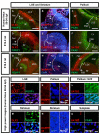
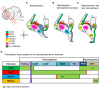
The subpallium comprises large parts of the basal ganglia including striatum and globus pallidus. Genes and factors involved in the development of the subpallium have been extensively studied in most vertebrates, including amphibians, birds, and rodents. However, our knowledge on patterning of the human subpallium remains insufficient. Using double fluorescent immunohistochemistry, we investigated the protein distribution of transcription factors involved in patterning of the subventricular zone (SVZ) in the human forebrain at late embryonic development. Furthermore, we compared the development of cortical and striatal precursors between human fetal brain and E14 and E16 fetal rat brains. Our results reveal that DLX2 marks SVZ precursors in the entire subpallium. Individual subpallial subdomains can be identified based on co-expression of DLX2 with either PAX6 or NKX2-1. SVZ precursors in the dorsal LGE and preopto-hypothalamic boundary are characterized by DLX2/PAX6 co-expression, while precursors in the MGE and preoptic region co-express DLX2/NKX2-1. SVZ precursors in the ventral LGE are DLX2(+)/PAX6(-)/NKX2-1(-). In terms of staging comparisons, the development of the corpus striatum in the human fetal brain during late embryonic stages corresponds well with the development of the striatum observed in E14 fetal rat brains. Our study demonstrates that the pattern underlying the development of the subpallium is highly conserved between rodents and humans and suggests a similar function for these factors in human brain development. Moreover, our data directly influence the application of ganglionic eminence derived human tissue for cell therapeutic approaches in neurodegenerative disorders such as Huntington's disease.
Keywords: development; ganglionic eminence; patterning; striatum; subpallium.
Figures

References
-
- Bayatti N., Moss J. A., Sun L., Ambrose P., Ward J. F. H., Lindsay S., et al. (2008). A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex N. Y. N 1991 18 1536–1548 10.1093/cercor/bhm184 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

