Structures and host-adhesion mechanisms of lactococcal siphophages
- PMID: 24474948
- PMCID: PMC3893620
- DOI: 10.3389/fmicb.2014.00003
Structures and host-adhesion mechanisms of lactococcal siphophages
Abstract
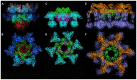
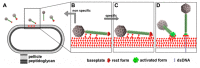
The Siphoviridae family of bacteriophages is the largest viral family on earth and comprises members infecting both bacteria and archaea. Lactococcal siphophages infect the Gram-positive bacterium Lactococcus lactis, which is widely used for industrial milk fermentation processes (e.g., cheese production). As a result, lactococcal phages have become one of the most thoroughly characterized class of phages from a genomic standpoint. They exhibit amazing and intriguing characteristics. First, each phage has a strict specificity toward a unique or a handful of L. lactis host strains. Second, most lactococcal phages possess a large organelle at their tail tip (termed the baseplate), bearing the receptor binding proteins (RBPs) and mediating host adsorption. The recent accumulation of structural and functional data revealed the modular structure of their building blocks, their different mechanisms of activation and the fine specificity of their RBPs. These results also illustrate similarities and differences between lactococcal Siphoviridae and Gram-negative infecting Myoviridae.
Keywords: Lactococcus lactis; Siphoviridae; bacteriophage; crystal structure; electron microscopy.
Figures









References
-
- Babu K. S., Spence W. S., Monteville M. R., Geller B. L. (1995). Characterization of a cloned gene (pip) from Lactococcus lactis required for phage infection. Dev. Biol. Stand. 85 569–575 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

